Abstract
The root dielectric response was measured on a minute scale to assess its efficiency for monitoring short-term cadmium (Cd) toxicity non-destructively. Electrical capacitance (CR), dissipation factor (DR) and electrical conductance (GR) were detected during the 24 to 168 h after Cd treatment (0, 20, 50 mg Cd2+ kg–1 substrate) in potted maize, cucumber and pea. Stress was also evaluated by measuring leaf chlorophyll content, Fv/Fm and stomatal conductance (gs) in situ, and shoot and root mass and total root length after harvest. CR showed a clear diurnal pattern, reflecting the water uptake rate, and decreased significantly in response to excessive Cd due to impeded root growth, the reduced tissue permittivity caused by accelerated lignification, and root ageing. Cd exposure markedly increased DR, indicating greater conductive energy loss due to oxidative membrane damage and enhanced electrolyte leakage. GR, which was coupled with root hydraulic conductance and varied diurnally, was increased transiently by Cd toxicity due to enhanced membrane permeability, but declined thereafter owing to stress-induced leaf senescence and transpiration loss. The time series of impedance components indicated the comparatively high Cd tolerance of the applied maize and the sensitivity of pea cultivar, which was confirmed by visible shoot symptoms, repeated physiological investigations and biomass measurements. The results demonstrated the potential of single-frequency dielectric measurements to follow certain aspects of the stress response of different species on a fine timescale without plant injury. The approach can be combined with widely used plant physiological methods and could contribute to breeding crop genotypes with improved stress tolerance.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In the plow-layer of agricultural soils, cadmium (Cd) usually ranges from 0.1 to 1 mg kg–1 worldwide, with mean of 0.4 mg kg–1, and rarely exceeds the 1–5 mg kg–1 maximum allowable concentration (McLaughlin et al. 2021). However, Cd concentration locally increases up to 10–40 mg kg–1 from geogenic sources, even reaches hundreds of mg kg–1 due to anthropogenic topsoil contamination (Kubier et al. 2019). Toxic levels of Cd accumulation in soils due to human activities, e.g. urban traffic, mining, industrial wastes, sewage sludge, phosphate fertilizers or agrochemicals, became a global problem (Bali et al. 2020). Cd is a non-essential heavy metal, and the highly soluble Cd2+ is taken up rapidly by plants and transferred to the food chain, threatening human health. The partitioning and mobility of Cd is regulated by the pH, colloidal and organic matter composition, cation exchange capacity and microbial activity of the soil (Bali and Sidhu 2022). Soil acidification induces Cd desorption from the binding sites, leading to enhanced availability of Cd2+ to plants. Cd excess induces several biochemical and physiological changes in plants, including disturbed water and nutrient uptake and transport, protein denaturation and reduced enzyme activity, oxidative damage caused by the generation of reactive oxygen species (ROS), chloroplast degradation, inhibited chlorophyll (Chl) biosynthesis and photochemical reactions, and decreased stomatal conductance (gs) and transpiration rate (Rizwan et al. 2016; Sahoo et al. 2022). These, in turn, can lead to visible toxicity symptoms, such as wilting, leaf roll, chlorosis and necrosis, as well as reduced organ growth, biomass and grain production. In general, Cd ions are mainly accumulated in the roots, where they impair membrane integrity (Pavlovkin et al. 2006), damage root tips, restrict root growth, and alter root anatomy (Lux et al. 2011). Root response is critical to plant stress tolerance, which varies with species, genotype, and growing conditions, and depends on the level, timing and duration of Cd exposure (Bali et al. 2020). Monitoring the root growth and function under heavy metal excess could facilitate the evaluation of plant sensitivity to Cd, and thus the identification of more tolerant crop cultivars (Akhtar et al. 2017). For this purpose, the application of dynamic, non-intrusive root methods, including electrical techniques, is preferable (Liu et al. 2021).
The polarization of root membranes changes the amplitude and phase of the input alternating current (AC) signal, and generates a measurable impedance response (Ehosioke et al. 2020). Thus, roots are considered analogous to cylindrical capacitors, in which the membranes, as dielectrics, store electric charges (Dalton 1995). The resultant capacitance (C) is directly proportional to the surface area (A) and the relative permittivity (εr), and inversely proportional to the thickness (d) of the membranes: C = ε0 × εr × A × d–1, where ε0 is the vacuum permittivity. In plant materials, electrical polarization occurs concurrently with electrical conduction: roots are leaky capacitors with high energy losses. They are equivalent to parallel resistance–capacitance (RC) circuits, comprising resistive symplastic and intercellular elements and capacitive membrane elements (Grimnes and Martinsen 2015). Lossy dielectrics have a complex relative permittivity: εr* = εrˊ– i × εr˝, where εrˊ is the real part (energy storage by polarization) and εr˝ is the imaginary part (energy dissipation by ionic conduction) of permittivity and i is the imaginary unit. Thus, the complex capacitance is: C* = ε0 × (εrˊ– i × εr˝) × A × d–1. The dissipation factor (D) is the ratio of dielectric losses to energy storage: D = εr˝/εrˊ = G/(ω × C), where G is the electrical conductance (= 1/R) and ω is the angular frequency. D is complementary to the phase angle (Φ) of impedance: D = tan(90°– Φ).
Root electrical capacitance (CR), measured between a ground electrode inserted into the growing medium, and a plant electrode fixed on the shoot base, was found to be linearly correlated with root system size in the case of the same species, substrate conditions and electrode locations (Chloupek et al. 2006; Středa et al. 2020). A few studies questioned whether dielectric properties were predictive for the whole root system due to the substantial current leakage at the proximal parts of the root–soil interface (Dietrich et al. 2012; Peruzzo et al. 2020). In contrast, others showed that the current could penetrate deep into the roots, so that most of the root system contributed to the impedance measured (Ozier-Lafontaine and Bajazet 2005; Ellis et al. 2013; Gu et al. 2021).
It is generally accepted that the electrical response depends not only on the size of the root system, but also on histological features (e.g. tissue density, water content, cell wall composition, lignification) related to the physiological status (Dalton 1995; Ellis et al. 2013; Peruzzo et al. 2020), indicating that CR can serve as an indicator of root functional intensity (Ellis et al. 2013; Ehosioke et al. 2020; Cseresnyés et al. 2024). Stress modifies membrane composition, and increases membrane permeability and thus the conductive dielectric loss (DR) in roots (Li et al. 2017; Jócsák et al. 2019). This appears as an increase in root electrical conductance (GR), which, as AC flows in the root–substrate continuum by ionic movement, is linked to root hydraulic conductance, LR (Weigand and Kemna 2019). For this reason, the dielectric characterization of plant tissues and organs has the potential to detect stress-related changes at the cellular level (Liu et al. 2021). Spectral (from Hz to MHz) impedance measurements were used to evaluate Cd toxicity in detached root segments of pea (Jócsák et al. 2010) and Cotinus (Xiang et al. 2018) seedlings. Only a few studies have used intact root systems for monitoring the effect of heavy metal, alkalinity or drought, but they all applied single-time measurements repeated once or twice a week on the same plants during their growth cycle (Vamerali et al. 2009; Cseresnyés et al. 2018, 2019). It is known, however, that Cd and other stressors induce faster changes in membrane surface charge density and electrical potential (Pavlovkin et al. 2006), and also in root apparent conductivity and polarization signatures (Weigand and Kemna 2019).
In this study, a pot experiment involving various species was undertaken to evaluate the usefulness of single-frequency (1 kHz) dielectric (CR, DR and GR) measurements in intact root–substrate systems in a minute-scale time resolution for monitoring the short-term effect of severe Cd toxicity. This time-series methodology necessitated the design and assembly of a specialized electrical measurement system. Non-destructive shoot investigations were also performed parallel to the electrical monitoring to follow the treatment efficiency via assessing the Cd-induced changes in photosynthetic and transpiration activities, and to demonstrate the relationship between the ongoing shoot physiological disruption and root dielectric response. The general aim was to present a novel in situ approach for tracking stress effects through the root system.
Materials and methods
Plant cultivation and treatment
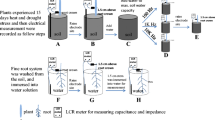
Maize (Zea mays L., cv. Mv Tarján), cucumber (Cucumis sativus L., cv. Perez-F1) and pea (Pisum sativum L., cv. Rhein dwarf) were used for the experiment. These plants are easily cultured in pots, and were reported to be convenient for toxicity tests, representing different sensitivity and response mechanisms to heavy metals (Moreno-Caselles et al. 2000, Rahoui et al. 2010, Akhtar et al. 2017). The plants were grown in 2.9 L plastic pots containing 1800 g of vermiculite–rhyolite 1.5:1 v/v mixture with a pH of 7.82, cation exchange capacity of 7.81 mmol 100 g–1 and 0.33 cm3 cm–3 water content at field capacity. The seeds were germinated on moistened paper towels in Petri dishes at 20 °C for 3 days in darkness, and were then planted one per pot to a depth of 1.5 cm. The plants were cultivated in a temperature- and light-controlled, 2.5 × 2.2 m growth chamber at 25/17 °C and 16/8 h light/dark cycles (light from 2 a.m. to 6 p.m.), and 60 ± 10% air humidity. Artificial illumination was provided by six metal halide lamps (Philips Master HPI-T Plus; Philips Lighting B.V., Eindhoven, The Netherlands) at about 600 μmol m2 s–1 (400–700 nm). The substrate was watered daily on a weight basis (± 1 g) to 80% of field capacity, and was fertilized twice a week with 150 mL of Hoagland’s solution. As the time-series dielectric measurement was only feasible for one plant at a time, a single seedling was cultivated per week to obtain a 28-day-old plant each week for the 7-day monitoring.
Altogether nine plants of each species were grown: three replicates for the three Cd treatments, namely control (Cd0), 20 mg Cd (Cd20) and 50 mg Cd (Cd50) kg–1 substrate. These high Cd levels were selected based on the preliminary tests (covered the range from 5 to 100 mg Cd kg–1) to provoke strong plant stress responses within the time frame of the dielectric measurement. Twenty-four hours before starting the electrical measurements, the substrate was irrigated with 250 mL of water (Cd0) or CdSO4 solution (Cd20 and Cd50).
Electrical measurements and plant harvest
As impedance parameters are very sensitive to substrate water content (SWC), the pot was placed in a tray filled with water at a steady depth of 6 mm right after the Cd treatment to adjust and maintain SWC constant during the entire measurement period. The water content in the bulk substrate (0–12 cm; the depth of the ground electrode) was 0.26 ± 0.01 cm3 cm–3, which was checked daily using a HS2 TDR meter attached to a CS659 probe (Campbell Inc, Logan, UT, USA). This procedure allowed the substrate surface to dry to 0.12 ± 0.01 cm3 cm–3 at 0–1 cm (checked with an MO750 meter; Extech Co. Ltd., Nashua, NH, USA) to minimize AC leakage (Gu et al. 2021). The root dielectric properties (modelled as a parallel RC circuit) were monitored at 1 kHz AC with 1 V terminal voltage, using a portable GW-Instek LCR-916 meter (Goodwill Co. Ltd., Taiwan) inside the growth chamber (Fig. 1a). The ground electrode was a stainless steel rod, 15 cm long and 6 mm i.d., inserted vertically into the growing medium to 12 cm depth, at a distance of 5 cm from the plant stem (Fig. 1b). The plant electrode was clamped 1 cm above the substrate through a 4 mm wide, 25 μm thick alumina strip that bent the stem. Although the low current density formed on root membranes by the AC signal does not damage plant functions (Jócsák et al. 2019), an asymmetric cycler (CRM-2H; ETI d.d., Izlake, Slovenia) was installed between the LCR terminal and the ground electrode (Cseresnyés et al. 2024). The cycler was set to a 0.5/5.5 min pulse/pause interval to interrupt the current flow generated continuously by the LCR instrument during operation. The impedance meter was connected to a laptop supplied with LCR900 v.1.201 data logging software (Fig. 1c). Each electrical monitoring started 24 h after the Cd treatment (this time was needed to stabilize SWC), in the middle of a light period (at 10 a.m.), and lasted for 144 h (until the 168th hour). Ten CR and DR data per hour (one during each pulse interval of the cycler) were recorded instrumentally throughout the time series. Five consecutive data (covering 30 min) were averaged to mitigate fluctuations. A GR value was calculated from each of the 288 data pairs, as CR × DR × ω.
After measurement the shoot was cut at the substrate surface. The roots were gently washed with running water and flotated to retain most of the fine roots, and were then thoroughly rinsed with distilled water three times to remove surface-bound ions. Shoot dry mass (SDM) and root dry mass (RDM) were determined after drying at 70 °C to constant weight, and root/shoot ratio (RSR) was calculated.
Elemental analysis
The dried shoots and roots were ground to a powder. Total-N concentration was measured by Kjeldahl’s method. The concentrations of Cd, P, K, Ca, Cu, Mg, Fe and Zn were analyzed with ICP-OES (iCAP™ 7400; Thermo Fisher Sci., Cambridge, UK).
Physiological investigations and root scanning
In order to avoid moving and touching the plants during the electrical measurements, another set of 27 plants (3 replicates × species × Cd treatment) were grown simultaneously for 28 days. The leaf Chl content, the maximum quantum efficiency of photosystem II (Fv/Fm) and gs were measured to evaluate plant physiological status. These are considered as sensitive parameters to assess the susceptibility of plants to stress, and can be easily monitored using handheld devices (Yaniccari et al. 2012). The leaf physiological measurements were taken in situ in the growth chamber during the mid-light hours (from 8 a.m. to 11 a.m.), right before (day 0 = D0), and 2, 4 and 7 days (D2, D4, D7) after Cd treatment. The measurements were performed on leaves 2–5 (numbered from the oldest to the youngest) for maize, 3–7 for cucumber and 2–6 for pea, avoiding leaf edges and major veins. The total Chl content (μmol m–2) was recorded as the mean of three readings on the upper (adaxial) side of the leaves, using an MC-100 instrument (Apogee Inc., Logan, UT, USA). Fv/Fm was determined with an OS-30p+ fluorometer (Opti-Sciences Inc., Hudson, NH, USA), by exposing the leaves to a saturation pulse of 6000 μmol m2 s–1 after 30 min of dark adaptation with black clips. The gs value (mmol m–2 s–1) was measured on the lower (abaxial) leaf surface with an SC-1 steady state diffusion porometer (Decagon Inc., Pullmann, WA, USA).
After the last set of measurements the shoots were cut and dried for SDM. The root systems were washed, stained with methylene blue solution for 24 h and rinsed with water. The total root length (RL) was determined with the modified line intersect method (Oliveira et al. 2000). Each root system was cut into 6–15 smaller subsamples (depending on root size). The subsample was placed in a thin layer of water in a rectangular glass tray over a regular grid of 1.0 cm squares. The roots were spread to minimize branch crossings and overlaps. The total number of intersections between the roots and the horizontal and vertical gridlines was counted, and root length was calculated as: root length (cm) = π/4 × number of intersects × grid unit (cm). The length of subsamples were summed up to obtain RL. Thereafter, RDM and RSR was determined.
Data analysis
The additive seasonal part was extracted from the time series of CR, DR and GR with STL seasonal trend decomposition based on locally estimated scatterplot smoothing, LOESS (Cleveland et al. 1990). Periodogram methods for spectral decomposition were applied in time series analysis (Shumway and Stoffer 2006). The 24-h seasonality was rejected if the s24 index was < 2, and accepted if s24 was ≥ 2. When seasonality was accepted, it was sufficient to check the trend component to test for monotonicity. When seasonality was rejected, a series of nonlinear kernel smoothing were performed with up to 24-h bandwidth, and monotonicity was only accepted if a monotonic curve was obtained after smoothing. Statistics were done using the “stl” function of R 4.0.5 (R Core Team 2021), the “periodogram” function of the “descomponer” package and the “sm.monotonicity” function of the “sm” package. The effect of Cd treatments on CR, DR and GR was analyzed by performing separate one-way ANOVA with Tukey’s post hoc test for data from each of the 288 time points. Normality, and the equality of variances in the data groups were examined with the Shapiro–Wilk test and Bartlett test, respectively.
One-way ANOVA with Tukey’s test was performed to evaluate the effect of Cd exposure on SDM, RDM, RL, RSR, and shoot and root element concentration. The nonparametric Kruskal–Wallis test with Dunn’s test was applied when the SD’s were significantly different. A repeated measures ANOVA with Tukey’s test was used to analyze the effect of Cd on Chl content, Fv/Fm and gs measured on various leaves. Statistical significance was assessed at p < 0.05 in each case.
Results
Time series of the dielectric properties
The periodogram method revealed a 24-h seasonality (s24: 3.87–8.26) in the data series of CR for each species and treatment, with a rapid increase and decrease at the beginning of each light and dark period, respectively (Fig. 2a, Table 1). According to the STL seasonal trend decomposition, the control (Cd0) plants exhibited a monotonous increasing trend in CR over time, up to 131%, 121% and 118% of the initial (24.5 h) CR value by the end of the measurement period (168 h) in the case of maize, cucumber and pea, respectively. The Cd20 plants also showed a monotonous increase in CR, but with a slower rate; the last CR values were 126%, 114% and 109% of those first measured for the above species. The trend, however, was found to be non-monotonic for the Cd50 treatments of all the species. In maize and cucumber, CR progressively increased until reaching a maximum at 141 h and 97 h, respectively, and then decreased to 109% and 102% of the initial value by the end of the measurement. In pea, there was no increasing trend in CR at all; almost the same peaks were measured during the first three mid-light periods around 24, 48 and 72 h, followed by a decrease to a lower level at 168 h (77% of the first CR value recorded). Compared to the Cd0 control, the Cd20 treatment had no significant effect on CR at any of the measurement time points for maize, but significantly reduced CR from 139 h onwards for cucumber, and much earlier, from 44 h for pea until the end of the monitoring period. The Cd50 treatment significantly decreased CR permanently from 121 h, 113 h and as early as 36.5 h for maize, cucumber and pea plants, respectively.
Time series of (a) root electrical capacitance (CR), (b) dissipation factor (DR) and (c) electrical conductance (GR) for maize, cucumber and pea exposed to different Cd levels (mean ± SD; n = 3). Cd0, Cd20 and Cd50: 0 (control), 20 and 50 mg Cd kg–1 substrate, respectively. Asterisks (green for Cd20 and red for Cd50) indicate the time point from which the Cd effect is significant at a given level compared to the control (obtained using one-way ANOVA with Tukey’s post hoc test). *p < 0.05, **p < 0.01, ***p < 0.001, NS non-significant again
The time series of DR showed significant 24-h seasonality only for control maize (s24: 2.83) and pea (s24: 2.61), with small local maxima during the mid-light periods (Fig. 2b, Table 1). A monotonous increasing trend in DR over time was found for each species and treatment. Depending on dosage, the addition of Cd provoked an increase in DR. The difference in DR from Cd0 controls became statistically significant from 165 h (Cd20) and 126.5 h (Cd50) for maize, from 96 h (Cd20) and 74 h (Cd50) for cucumber, and right from the beginning of the dielectric measurement (24.5 h) for both Cd treatments on pea.
Each data series of GR showed significant 24-h seasonality (s24: 3.82–8.84; Fig. 2c, Table 1), which evidently corresponded with the light/dark variations of CR. The trend in GR was always monotonously increasing, except for the Cd50 treatment on pea. In the latter case there was no monotonic trend in the series: after a sharp increase, GR reached a plateau around 117 h, and then began to drop markedly. Compared to the controls, the Cd20 treatment did not significantly influence GR at any time point for maize, but increased it transitionally (from 129 to 160 h) for cucumber, and from 105.5 h until the end of the measurements for pea. The Cd50 treatment only resulted in a significant increase in GR for a short period of time (from 153.5 to 160 h) for maize, but permanently from 81.5 h for cucumber, and from 45.5 to 156 h for pea plants.
Shoot symptoms and physiological response
Obvious toxicity symptoms appeared on the aboveground plant parts by the end of the 7-day Cd exposure. Maize leaves began to curl, and turned yellow, chiefly on the tips and edges. The lower leaves of cucumber were slightly wilted, and became chlorotic around the main veins. The most severe damage was visible in pea: each leaf and the shoot tip wilted, and the two or three oldest leaves dried up.
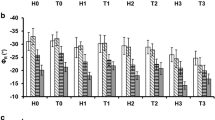
Cd toxicity was manifested as reduced leaf Chl content, Fv/Fm and gs, particularly in pea (Fig. 3). The Chl concentration in maize leaves only decreased significantly at the higher Cd level at D7, compared to the controls. A quicker, stronger response to Cd was observed in cucumber and pea: the Chl content significantly decreased from D4 and from D2 in the Cd20 and Cd50 treatments, respectively. The stress-induced reduction in Fv/Fm proved to be statistically significant only for the Cd50 treatment on pea, but from D2 onwards in this case. Cd exposure had a marked negative effect on gs: the decrease under stress was significant at D7 for Cd20 and at D4 and D7 for Cd50 maize plants, compared to the Cd0 controls, but both Cd levels resulted in a significant impact already from D2 onwards in cucumber and pea.
(a) Changes in the total chlorophyll (Chl) content, (b) maximum quantum efficiency of photosystem II (Fv/Fm) and (c) stomatal conductance (gs) over time (DAT: days after treatment) in maize, cucumber and pea plants exposed to different Cd levels. Cd0, Cd20 and Cd50: 0 (control), 20 and 50 mg Cd kg–1 substrate, respectively. The values were averaged across leaves and replicate plants (mean ± SD; n = 3) for clarity. Asterisks (green for Cd20 and red for Cd50) represent significant differences compared to the control (obtained using repeated-measures ANOVA with Tukey’s test). *p < 0.05, **p < 0.01, ***p < 0.001
Shoot and root biomass and total root length
Cd addition had no significant influence on the SDM, RDM, RL or RSR of maize (Fig. 4). However, the Cd50 treatment led to a non-significant, 13% and 23% decrease in RDM and RL, respectively, compared to the Cd0 controls. In cucumber, the effect of the low Cd level on shoot and root growth proved to be statistically insignificant, whereas the high Cd dose resulted in a significant, 16%, 23% and 31% decrease in SDM, RDM and RL, respectively. RSR significantly reduced by both Cd treatments. Among the species, the biomass production of pea was the most affected by Cd stress: compared to the control plants, the Cd20 treatment significantly reduced SDM by 17%, RDM by 19% and RL by 25%, while the corresponding changes were 41%, 37% and 44% in the case of the Cd50 treatment. No significant changes in RSR by Cd was found.
(a) Shoot dry mass (SDM; mean ± SD; n = 6), (b) root dry mass (RDM; n = 6), (c) total root length (RL; n = 3) and (d) root/shoot ratio (RSR; n = 6) for maize, cucumber and pea exposed to different Cd levels for 7 days. Cd0, Cd20 and Cd50: 0 (control), 20 and 50 mg Cd kg–1 substrate, respectively. Asterisks represent significant differences from the controls obtained using one-way ANOVA with Tukey’s test. *p < 0.05, **p < 0.01, ***p < 0.001, NS non-significant
Shoot and root element concentration
ICP-OES analysis demonstrated a highly significant increase in the shoot and root Cd concentration at increasingly high Cd treatment levels (Fig. 5). The Cd concentration in dry shoot biomass reached 17.9, 38.1 and 33.6 mg kg–1 in the Cd50 treatments of maize, cucumber and pea, respectively, compared to the 1.6–1.9 mg kg–1 values measured in the control plants. A substantially higher amount of Cd was accumulated in the roots: 140, 889 and 470 mg kg–1 Cd was determined in Cd50 maize, cucumber and pea, respectively, compared to 2.2–5.0 mg kg–1 Cd found in the Cd0 controls. Both the Cd doses significantly decreased the concentration of all the investigated elements except P in pea root, and reduced the N, P, K, Cu and Zn in the shoot (Table S1). In cucumber the effect was only significant for Ca, Cu, Fe and Zn, whereas the element concentration of maize root was not affected statistically by the Cd treatments (it should be noted that the low replicate number may have limited the level of significance in many cases).
Cd concentrations (mean ± SD; n = 3) in dry (a) shoot and (b) root samples of maize, cucumber and pea exposed to different Cd levels for 7 days. Cd0, Cd20 and Cd50: 0 (control), 20 and 50 mg Cd kg–1 substrate, respectively. ***: significantly different from the controls at the p < 0.001 level, obtained using the nonparametric Kruskal–Wallis test with Dunn’s test
Discussion
Root dielectric response to Cd
Cell membrane capacitors were reported to show constant or slightly decreasing C with rising temperature due to increasing polarization loss (Grimnes and Martinsen 2015). Therefore, the marked 24-h seasonality in the CR data series was attributed to the cyclic root water uptake activity strongly linked to the light/dark changes in canopy transpiration (Henzler et al. 1999), as was previously verified by modifying temperature regime and light cycles under chamber conditions (Cseresnyés et al. 2024). Diurnal variation in plant hydraulics is driven by endogenous circadian regulation coincidently with external temperature and photoperiod cues (Greenham and McClung 2015). The present experimental results supported the previous hypothesis that CR is influenced by both the root functional status and the geometrical size (Dalton 1995; Ehosioke et al. 2020). The increasing trend in CR indicated an extension of the root system, especially for maize maintaining intensive vegetative growth. Depending on the level applied, Cd significantly reduced CR compared to the control plants, and interrupted the increasing trend in the time series. Cd excess in the rhizosphere impedes root cell division and root-hair production, and inhibits root initiation and elongation, leading to the formation of shorter, thicker root branches (Lux et al. 2011). Accelerated exo- and endodermal maturation and the deposition of lignin and suberin closer to the root apices, together with an increase in epidermal and cortical thickness provide a more efficient barrier to Cd transport into the vascular cylinder and thus to the shoot (Hose et al. 2001; Díaz et al. 2021). Basically, there are three reasons for the Cd-induced reduction in the magnitude of CR. First, Cd decreased RL and the root surface area, i.e. the area of the polarizable membrane dielectrics (A) in the capacitor. Second, lignin and suberin have much smaller εr (from 2 to 2.4) than the other main root constituents, in particular cellulose (εr ~ 7.6) and water (εr ~ 80) (Ellis et al. 2013). Enhanced suberization, and the lower root water content, which is another typical consequence of Cd toxicity (Xiang et al. 2018) or soil alkalinity (Cseresnyés et al. 2019), decrease the resultant εr of root tissue. Third, restricted root elongation reduced the ratio of young, absorptive root parts to older, more suberized transporting segments, leading to a lower integrated water uptake rate in the stressed root system (Lobet et al. 2013).
In some cases, DR values increased over time up to the mid-light hours. Depending on histological features and environmental conditions, water flows across the root cylinder through a variable combination of hydrostatically driven apoplastic, and mainly osmotically controlled (aquaporin-mediated) cell-to-cell (i.e. symplastic and transmembrane) pathways (Sivasakthi et al. 2020). During daylight the higher transpiration pull and xylem tension increase the ratio of the primarily electrically resistive apoplastic flow to the more capacitive cell-to-cell movement (Lobet et al. 2013), leading to enhanced conductive energy loss, DR. However, the present results for cucumber question the efficiency of using dielectric measurements to reveal clear diurnal cycles in DR under the experimental conditions applied here. In Cd-treated plants, enhanced leaf senescence and reduced light transpiration (detected via gs) was thought to be responsible for the cessation of seasonality in the DR time series. Although tissue development during root ageing always causes some increase in DR detected at 1 kHz AC (Ehosioke et al. 2023), more intensive changes were observed when plants were exposed to various substrate Cd levels (Cseresnyés et al. 2018). Cd toxicity triggers rapid membrane depolarization in the outer cortical cells, and induces membrane lipid peroxidation due to excessive ROS production, altering membrane structure and fluidity (Artiushenko et al. 2014). Depolarization by the Cd levels applied was accompanied by a marked increase in membrane hydraulic conductivity and by substantial electrolyte leakage from the cells even 24–48 h after treatment in maize (Pavlovkin et al. 2006), cucumber (Kabała et al. 2008) and pea roots (Lehotai et al. 2011), as was demonstrated in the present study by significantly higher DR values. Enhanced membrane permeability, the death of root cortical cells and the resulting H2O2-induced aerenchyma formation caused by Cd toxicity was reported to modify the impedance response, manifested as reduced intracellular resistance (Xiang et al. 2018) or Φ (Ehosioke et al. 2023).
The light/dark pattern in the GR time series is likely linked to the fluctuation of LR (Weigand and Kemna 2019), which is coincident with the diurnal oscillation of aquaporin abundance and also of the stomatal closure and transpiration rate (Henzler et al. 1999). Notably, increasing temperature, per se, increases electrolyte G, but light/dark cycles in GR have also been observed previously under constant temperature (yet unpublished results). Although the restricted root development caused by Cd treatment was over-compensated by the enhanced electrical conductivity of the disrupted membranes (Rahoui et al. 2010), leading to a transient increase in GR, the measured values began to decrease thereafter due to accelerated senescence and the loss of transpiration, principally for pea.
Physiological and growth responses in relation to Cd tolerance
Besides the impedance response detected, the visible toxicity symptoms on the shoot, the leaf physiological traits, and the SDM, RDM and RL measurements clearly indicated the serious adverse effect of the two Cd doses applied. Previous studies on maize (Akhtar et al. 2017), cucumber (Moreno-Caselles et al. 2000) and pea (Hattab et al. 2009) reported that an increase in the Cd dose added to the medium of developing plants provoked a gradual decline in these parameters, and that the effect became more pronounced with the exposition time. Furthermore, in accordance with the present findings, gs showed a more rapid and prominent response to Cd stress, followed by a reduction in Chl concentration and later in Fv/Fm (Wang et al. 2009; Chaneva et al. 2010). The quick stomatal closure and transpiration loss, and the consequent reduced water uptake rate were convincingly demonstrated by the CR and GR data series. A much higher amount of Cd was found in the roots than in the shoots. This was due to the binding and retention of Cd2+ by negatively charged, strongly suberized cell walls, and also to the chelation and sequestration of metal ions into the vacuoles (Lux et al. 2011). Plants evolved these defense mechanisms to restrict the radial movement of Cd to xylem vessels to protect shoots from Cd loads (Hose et al. 2001).
Root dielectric responses clearly indicated differences in Cd sensitivity between the studied species. The CR and GR time series themselves showed that pea was the most rapidly and seriously impacted by Cd excess: the stressed plants exhibited the highest relative decrease in CR compared to the controls by the end of treatment (Table S2), with a significant treatment effect for both metal levels. Supporting this finding, the degree of visible shoot damage, and the percentage changes in physiological and biomass parameters were the greatest for pea among the three species. Being an important food crop, pea is widely used in pollution tests due to its high sensitivity to Cd (Hattab et al. 2009; Rahoui et al. 2010). It has been previously reported for pea that a week of Cd exposure resulted in substantial endodermal suberin deposition and lamellae formation, a reduced number and length of lateral roots, and characteristic root browning (Rodríguez-Serrano et al. 2006; Głowacka et al. 2019). These results concur with the present dielectric monitoring, and with post-harvest root measurements and visual observations. Although root architecture has not been evaluated, the higher percentage reduction in RL than in RDM implied that Cd inhibited lateral root initiation and elongation.
Cucumber seemed less susceptible to stress: the Cd loads were observed to have somewhat smaller impacts on dielectric, physiological and growth responses, with significant differences mainly in the Cd50 treatment vs. controls. However, fast stomatal closure was detected for the Cd20 treatment as well, in agreement with earlier literature (Sun et al. 2015). Maize proved to be the most Cd-tolerant plant on the basis of the latest stress response and the lowest relative change both in CR and in the other measured properties. Maize is, in fact, known to be a promising Cd-accumulator crop with the ability to tolerate up to 45 mg Cd per kg root dry weight without exhibiting visible injury symptoms, although the tolerance level to Cd and other soil-borne stresses varies greatly among genotypes (Akhtar et al. 2017; Klimešová et al. 2020).
The macro- and micronutrient analysis of shoot and root samples confirmed the different Cd sensitivity levels of the plant species tested. Cd excess triggers a rapid K+ efflux from the cells through damaged membranes (Pavlovkin et al. 2006), and was found to displace Ca2+ from the cell walls (Lehotai et al. 2011). By competing for the same plasma membrane transporters, Cd reduces the uptake and transport of many elements, including N, P, K, Ca, Cu, Fe, Mg, Mn and Zn, leading to serious nutrient deficiency and imbalance in plants (Rizwan et al. 2016). In the present case, shoot and root element composition was influenced by Cd excess to the greatest and least extent in pea (sensitive) and maize (tolerant), respectively (Table S1).
Conclusions and prospects
Low-frequency (1 kHz) dielectric monitoring with fine time resolution was performed over a 6-day period in intact root–substrate systems, using a specialized two-terminal measurement set-up on different plants under chamber conditions. The experimental results supported the hypothesis that this novel impedance measurement technique was suitable for tracking real-time changes, including diurnal patterns in root water uptake activity in situ. Moreover, this non-invasive method proved to be a reliable diagnostic tool to monitor the short-term response of plants to stress targeting the roots, such as the addition of Cd to the growing medium. The different susceptibility of the studied species to various substrate Cd levels could also be evaluated dynamically. The single-frequency dielectric monitoring is possible using a cheap handheld LCR device. Nevertheless, maintenance of stable SWC with partially dried surface (to avoid current leakage at the root neck) is an important limitation of the method application. Although impedance parameters per se serve as indicators of some aspects of root histological and functional changes, the dielectric approach can also be easily integrated into conventional and advanced plant physiological investigations. Not only heavy metals but other root stress factors, such as nutrient deficiencies, anomalies in soil temperature and pH, agrochemicals and various soil pollutants, can be potentially evaluated in situ without affecting plant life functions. From an agricultural point of view, the present method could promote the screening and selection of crop genotypes with improved stress tolerance and consequently yield potential. Furthermore, the dielectric assessment of tolerance and acclimatory response to heavy metal pollution may help in evaluating the phytoremediation potential of plants, contributing to more efficient practical use. Although single-time impedance measurements have been performed successfully for several field-grown crops, the adaptation of the continuous monitoring presented here to field conditions (i.e. variable soil moisture) will be a challenging task requiring much time and effort.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- A:
-
Capacitor plate area
- AC:
-
Alternating current
- C:
-
Electrical capacitance
- Chl:
-
Chlorophyll
- CR :
-
Electrical capacitance of root–substrate system
- d:
-
Capacitor plate separation
- D:
-
Dissipation factor
- DR :
-
Dissipation factor of root–substrate system
- Fv/Fm :
-
Maximum quantum yield of PSII photochemistry
- G:
-
Electrical conductance
- GR :
-
Electrical conductance of root–substrate system
- gs :
-
Stomatal conductance
- LR :
-
Root hydraulic conductance
- R:
-
Electrical resistance
- RDM:
-
Root dry mass
- RL:
-
Total root length
- ROS:
-
Reactive oxygen species
- RSR:
-
Root/shoot ratio
- SDM:
-
Shoot dry mass
- SWC:
-
Substrate water content
- ε 0 :
-
Vacuum permittivity
- ε r :
-
Relative permittivity
- Φ :
-
Impedance phase angle
- ω :
-
Angular frequency
References
Akhtar T, Zia-ur-Rehman M, Naeem A, Nawaz R, Ali S, Murtaza G, Maqsood MA, Azhar M, Khalid H, Rizwan M (2017) Photosynthesis and growth response of maize (Zea mays L.) hybrids exposed to cadmium stress. Environ Sci Pollut Res 24:5521–5529. https://doi.org/10.1007/s11356-016-8246-0
Artiushenko T, Syshchykov D, Gryshko V, Čiamporová M, Fiala R, Repka V, Martinka M, Pavlovkin J (2014) Metal uptake, antioxidant status and membrane potential in maize roots exposed to cadmium and nickel. Biologia 69:1142–1147. https://doi.org/10.2478/s11756-014-0414-4
Bali AS, Sidhu GPS (2022) Cadmium uptake, toxicity, and tolerance in plants. In: Aftab T, Hakeem KR (eds) Heavy Metal Toxicity in Plants. Physiological and Molecular Adaptations. CRC Press, Boca Raton, pp 193–206. https://doi.org/10.1201/9781003155089-3
Bali AS, Sidhu GPS, Kumar V (2020) Root exudates ameliorate cadmium tolerance in plants: a review. Environ Chem Lett 18:1243–1275. https://doi.org/10.1007/s10311-020-01012-x
Chaneva G, Parvanova P, Tzvetkova N, Uzunova A (2010) Photosynthetic response of maize plants against cadmium and paraquat impact. Water Air Soil Poll 208:287–293. https://doi.org/10.1007/s11270-009-0166-x
Chloupek O, Forster BP, Thomas WTB (2006) The effect of semi-dwarf genes on root system size in field-grown barley. Theor Appl Genet 112:779–786. https://doi.org/10.1007/s00122-005-0147-4
Cleveland RB, Cleveland WS, McRae JE, Terpenning I (1990) STL: A seasonal-trend decomposition. J Offic Stat 6:3–73
Cseresnyés I, Rajkai K, Takács T, Vozáry E (2018) Electrical impedance phase angle as an indicator of plant root stress. Biosyst Eng 169:226–232. https://doi.org/10.1016/j.biosystemseng.2018.03.004
Cseresnyés I, Takács T, Sepovics B, Kovács R, Füzy A, Parádi I, Rajkai K (2019) Electrical characterization of the root system: a noninvasive approach to study plant stress responses. Acta Physiol Plant 41:169. https://doi.org/10.1007/s11738-019-2959-x
Cseresnyés I, Füzy A, Kabos S, Kelemen B, Rajkai K, Takács T (2024) Monitoring of plant water uptake by measuring root dielectric properties on a fine timescale: diurnal changes and response to leaf excision. Plant Methods 20:5. https://doi.org/10.1186/s13007-023-1133-8
Dalton FN (1995) In-situ root extent measurements by electrical capacitance methods. Plant Soil 173:157–165. https://doi.org/10.1007/BF00155527
Díaz AS, da Cunha Cruz Y, Duarte VP, de Castro EM, Magalhães PC, Pereira FJ (2021) The role of reactive oxygen species and nitric oxide in the formation of root cortical aerenchyma under cadmium contamination. Physiol Plant 173:2323–2333. https://doi.org/10.1111/ppl.13582
Dietrich RC, Bengough AG, Jones HG, White PJ (2012) A new physical interpretation of plant root capacitance. J Exp Bot 63:6149–6159. https://doi.org/10.1093/jxb/ers264
Ehosioke S, Nguyen F, Rao S, Kremer T, Placencia-Gomez E, Huisman JA, Kemna A, Javaux M, Garré S (2020) Sensing the electrical properties of roots: A review. Vadose Zone J 19:e20082. https://doi.org/10.1002/vzj2.20082
Ehosioke S, Garré S, Huisman JA, Zimmermann E, Placencia-Gomez E, Javaux M, Nguyen F (2023) Spectroscopic approach toward unraveling the electrical signature of roots. J Geophys Res-Biogeo 128:e2022JG007281. https://doi.org/10.1029/2022JG007281
Ellis T, Murray W, Kavalieris L (2013) Electrical capacitance of bean (Vicia faba) root systems was related to tissue density – a test for the Dalton Model. Plant Soil 366:575–584. https://doi.org/10.1007/s11104-012-1424-z
Głowacka K, Źróbek-Sokolnik A, Okorski A, Najdzion J (2019) The effect of cadmium on the activity of stress-related enzymes and the ultrastructure of pea roots. Plants 8:413. https://doi.org/10.3390/plants8100413
Greenham K, McClung CR (2015) Integrating circadian dynamics with physiological processes in plants. Nat Rev Genet 16:598–610. https://doi.org/10.1038/nrg3976
Grimnes S, Martinsen ØG (2015) Bioimpedance and Bioelectricity Basics, 3rd edn. Academic Press, Oxford. https://doi.org/10.1016/C2012-0-06951-7
Gu H, Liu L, Butnor JR, Sun H, Zhang X, Li C, Liu X (2021) Electrical capacitance estimates crop root traits best under dry conditions – a case study in cotton (Gossypium hirsutum L.). Plant Soil 467:549–567. https://doi.org/10.1007/s11104-021-05094-6
Hattab S, Dridi B, Chouba L, Kheder MB, Bousetta H (2009) Photosynthesis and growth responses of pea Pisum sativum L. under heavy metal stress. J Environ Sci 21:1552–1556. https://doi.org/10.1016/S1001-0742(08)62454-7
Henzler T, Waterhouse RN, Smyth AJ, Carvajal M, Cooke DT, Schäffner AR, Steudle E, Clarkson DT (1999) Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus. Planta 210:50–60. https://doi.org/10.1007/s004250050653
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001) The exodermis: a variable apoplasic barrier. J Exp Bot 52:2245–2264. https://doi.org/10.1093/jexbot/52.365.2245
Jócsák I, Droppa M, Horváth G, Bóka K, Vozáry E (2010) Cadmium- and flood-induced anoxia stress in pea roots measured by electrical impedance. Z Naturforsch C 65:95–102. https://doi.org/10.1515/znc-2010-1-216
Jócsák I, Végvári G, Vozáry E (2019) Electrical impedance measurement on plants: a review with some insights to other fields. Theor Exp Plant Physiol 31:359–375. https://doi.org/10.1007/s40626-019-00152-y
Kabała K, Janicka-Russak M, Burzyński M, Kłobus G (2008) Comparison of heavy metal effect on the proton pumps of plasma membrane and tonoplast in cucumber root cells. J Plant Physiol 165:278–288. https://doi.org/10.1016/j.jplph.2007.03.007
Klimešová J, Holková L, Středa T (2020) Drought stress response in maize: molecular, morphological and physiological analysis of tolerant and sensitive genotypes. Maydica 65:1264. https://journals-crea.4science.it/index.php/maydica/article/view/2024/1264. Accessed 26 Feb 2024
Kubier A, Wilkin RT, Pichler T (2019) Cadmium in soils and groundwater: a review. Appl Geochem 108:104388. https://doi.org/10.1016/j.apgeochem.2019.104388
Lehotai N, Pető A, Bajkán S, Erdei L, Tari I, Kolbert Z (2011) In vivo and in situ visualization of early physiological events induced by heavy metals in pea root meristem. Acta Physiol Plant 33:2199–2207. https://doi.org/10.1007/s11738-011-0759-z
Li MQ, Li JY, Wei XH, Zhu WJ (2017) Early diagnosis and monitoring of nitrogen nutrition stress in tomato leaves using electrical impedance spectroscopy. Int J Agr Biol Eng 10:194–205. https://doi.org/10.3965/j.ijabe.20171003.3188
Liu Y, Li DM, Qian J, Di B, Zhang G, Ren ZH (2021) Electrical impedance spectroscopy (EIS) in plant root research: a review. Plant Methods 17:118. https://doi.org/10.1186/s13007-021-00817-3
Lobet G, Hachez C, Chaumont F, Javaux M, Draye X (2013) Root water uptake and water flow in the root–soil domain. In: Eshel A, Beeckman T (eds) Plant roots: The hidden half, 4th edn. CRC Press, Boca Raton, p 24/1–18. https://doi.org/10.1201/b14550
Lux A, Martinka M, Vaculík M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37. https://doi.org/10.1093/jxb/erq281
McLaughlin MJ, Smolders E, Zhao FJ, Grant C, Montalvo D (2021) Managing cadmium in agricultural systems. Adv Agron 166:1–129. https://doi.org/10.1016/bs.agron.2020.10.004
Moreno-Caselles J, Moral R, Pérez-Espinosa A, Pérez-Murcia MD (2000) Cadmium accumulation and distribution in cucumber plant. J Plant Nutr 23:243–250. https://doi.org/10.1080/01904160009382011
Oliveira MRG, van Noordwijk M, Gaze SR, Brouwer G, Bona S, Mosca G, Hairiah K (2000) Auger sampling, ingrowth cores and pinboard methods. In: Smit AL, Bengough AG, Engels C, van Noordwijk M, Pellerin S, van de Geijn SC (eds) Root Methods: A Handbook. Springer, Berlin, pp 175–210. https://doi.org/10.1007/978-3-662-04188-8
Ozier-Lafontaine H, Bajazet T (2005) Analysis of root growth by impedance spectroscopy (EIS). Plant Soil 277:299–313. https://doi.org/10.1007/s11104-005-7531-3
Pavlovkin J, Luxová M, Mistríková I, Mistrík I (2006) Short- and long-term effects of cadmium on transmembrane electric potential (Em) in maize roots. Biologia 61:109–114. https://doi.org/10.2478/s11756-006-0016-x
Peruzzo L, Chou C, Wu Y, Schmutz M, Mary B, Wagner FM, Petrov P, Newman G, Blancaflor EB, Liu X, Ma X, Hubbard S (2020) Imaging of plant current pathways for non-invasive root phenotyping using a newly developed electrical current source density approach. Plant Soil 450:567–584. https://doi.org/10.1007/s11104-020-04529-w
R Core Team (2021) A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/. Accessed 5 Sept 2021
Rahoui S, Chaoui A, El Ferjani E (2010) Membrane damage and solute leakage from germinating pea seed under cadmium stress. J Hazard Mater 178:1128–1131. https://doi.org/10.1016/j.jhazmat.2010.01.115
Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016) Cadmium minimization in wheat: A critical review. Ecotox Environ Saf 130:43–53. https://doi.org/10.1016/j.ecoenv.2016.04.001
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544. https://doi.org/10.1111/j.1365-3040.2006.01531.x
Sahoo RK, Pradhan M, Palei M, Maitra S (2022) Responses and adaptation of photosynthesis and respiration under heavy metal stress. In: Aftab T, Hakeem KR (eds) Heavy Metal Toxicity in Plants. Physiological and Molecular Adaptations. CRC Press, Boca Raton, pp 27–33. https://doi.org/10.1201/9781003155089-3
Shumway RH, Stoffer DS (2006) Time Series Analysis and Its Applications, 3rd edn. Springer, New York. https://doi.org/10.1007/0-387-36276-2
Sivasakthi K, Tharanya M, Zaman-Allah M, Kholová J, Thirunalasundari T, Vadez V (2020) Transpiration difference under high evaporative demand in chickpea (Cicer arietinum L.) may be explained by differences in the water transport pathway in the root cylinder. Plant Biol 22:769–780. https://doi.org/10.1111/plb.13147
Středa T, Haberle J, Klimešová J, Klimek-Kopyra A, Středová H, Bodner G, Chloupek O (2020) Field phenotyping of plant roots by electrical capacitance – a standardized methodological protocol for application in plant breeding: a review. Int Agrophys 34:173–184. https://doi.org/10.31545/intagr/117622
Sun S, Li M, Zuo J, Jiang W, Liu D (2015) Cadmium effects on mineral accumulation, antioxidant defense system and gas exchange in cucumber. Zemdirbyste 102:193–200. https://doi.org/10.13080/z-a.2015.102.025
Vamerali T, Bandiera M, Coletto L, Zanetti F, Dickinson NM, Mosca G (2009) Phytoremediation trials on metal- and arsenic-contaminated pyrite wastes (Torviscosa, Italy). Environ Pollut 157:887–894. https://doi.org/10.1016/j.envpol.2008.11.003
Wang H, Zhao SC, Liu RL, Zhou W, Jin JY (2009) Changes of photosynthetic activities of maize (Zea mays L.) seedlings in response to cadmium stress. Photosynthetica 47:277–283. https://doi.org/10.1007/s11099-009-0043-2
Weigand M, Kemna A (2019) Imaging and functional characterization of crop root systems using spectroscopic electrical impedance measurements. Plant Soil 435:201–224. https://doi.org/10.1007/s11104-018-3867-3
Xiang D, Zhang G, Gong R, Di B, Tian Y (2018) Effect of cadmium stress on growth and electrical impedance spectroscopy parameters of Cotinus coggygria roots. Water Air Soil Pollut 229:279. https://doi.org/10.1007/s11270-018-3924-9
Yaniccari M, Tambussi E, Istilart C, Castro AM (2012) Glyphosate effects on gas exchange and chlorophyll fluorescence responses of two Lolium perenne L. biotypes with different herbicide sensitivity. Plant Physiol Biochem 57:210–217. https://doi.org/10.1016/j.plaphy.2012.05.027
Acknowledgements
The authors thank Gábor Nemes and Károly Podráczki (Colas Co. Ltd.) for ensuring the plant growing substrate, Anna Cseresnyés for plant germination, Sándor Kabos for helping in statistical analysis, József Monori for assembling the electrical measurement circuit, Barbara Hooper for language editing, and Prof. Kálmán Rajkai for their valuable remarks.
Funding
Open access funding provided by HUN-REN Centre for Agricultural Research. The project was funded by the National Research, Development and Innovation Fund of Hungary (NKFIH), project No. 137617, financed under the FK-21 funding scheme.
Author information
Authors and Affiliations
Contributions
Imre Cseresnyés conceptualized the research, performed the dielectric measurements, interpreted the data, read the relevant literature and drafted the paper. Tünde Takács maintained the plants, completed the shoot measurements and interpreted the data. Anna Füzy performed the statistical analysis and reviewed the paper. All the authors improved the manuscript critically, and approved the submission.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Elena Maestri
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cseresnyés, I., Takács, T. & Füzy, A. Detection of plant cadmium toxicity by monitoring dielectric response of intact root systems on a fine timescale. Environ Sci Pollut Res 31, 30555–30568 (2024). https://doi.org/10.1007/s11356-024-33279-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33279-w