Abstract
Freshwater scarcity, salinity, and poor soil fertility are the major challenges affecting both food and feed productions in arid and semi-arid regions of the world. Utilization of brackish water in the production of saline-tolerant fish and valuable field crops under an integrated system is promising in the maximization of yield per crop. The aim of this study, therefore, was to (1) assess the effect of saline aquaculture wastewater on the growth, yield, forage quality, and nutritive composition of sorghum seeds and (2) assess the effect of different water qualities on the survival, growth performance, and health status of Pangasianodon hypophthalmus. The experiment was conducted in a randomized completely block design of four salinity treatments with three replicates, i.e., control (freshwater mixed with inorganic fertilizers), 5000 ppm, 10,000 ppm, and 15,000 ppm. Our results indicated that although the control exhibited the highest growth (plant height, leaf number, internode number, leaf area, and soil–plant analysis development), grain, and forage yield, no significant differences were noted among the treatments. Likewise, no significant difference in the grain nutrient composition was noted among all the treatments. Assessment of the forage quality revealed improved crude protein content in the control compared to the saline treatments. However, no significant differences in the leaves and stalks fiber fractions were noted among all the treatments. Furthermore, rumen fermentation in terms of in vitro digestibility indicated no significant differences in the in vitro digestible dry matter, digestible organic matter, metabolic energy, net energy, microbial protein, short-chain fatty acids, and total dissolved nutrients among the treatments. However, rearing P. hypophthalmus in water salinities exceeding 10,000 ppm reduced the growth performance and health status of fish. Therefore, the integration of sorghum and P. hypophthalmus production in water salinities not exceeding 5000 ppm is a viable alternative to maximize brackish water productivity in freshwater-scarce regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate change has altered the global weather and water patterns leading to severe droughts, water shortages, and increasing salinity in several regions of the world (Ismail and Go 2021). According to the United Nations Sustainable Development (UNSDG), half of the world’s population has already experienced severe water scarcity for at least one month in a year, and by 2030, it is expected that ~ 700 million people will be affected by severe water scarcity (Ismail and Go 2021). This is attributed to the rapid growth in human population coupled with the increasing water demand and usage which together will put more pressure on the available limited freshwater resources (Huang et al. 2012; Arnell 2018; Mishra et al. 2021; Kumar et al. 2021). For example, Egypt, one of the most populous countries in Africa, is currently facing severe water shortages and her water poverty threshold has reached less than 500 m3 of water per capita per year (Rashid 2012). Moreover, the recent filling and operation of the Grand Ethiopian Renaissance Dam have further threatened Egypt’s freshwater supply, food security, and economic development. In the same regard, the over-dependence of the country’s agricultural sector on synthetic fertilizers and underground water for irrigation of water-intensive crops such as wheat, corn, and rice has further lowered crop yields due to the increasing soil salinity and land degradation (Awaad et al. 2010; Abd El-Gawad and Morsy 2017; Ding et al. 2020; Moghazy and Kaluarachchi 2020). To overcome the aforementioned challenges, there is a need for the implementation of realistic and scientifically feasible interventions to safeguard the country’s water and food security.
Maximization of water productivity through the introduction of salt and drought-tolerant crops to the Egyptian cropping patterns will aid in tackling crop yield losses due to the increasing soil salinity and freshwater scarcity (El Demerdash et al. 2022). Moreover, the integration of salt-tolerant crops with aquaculture (i.e., Integrated Aqua-Agriculture Systems [IAAS]) will promote more crop (i.e., plant and fish) yield per drop thus contributing to double economic benefits. IAAS operates on a principle of wastewater reuse where aquaculture effluents are utilized as both irrigation water and nutrient sources for plants (Farrag et al. 2021; Sewilam et al. 2022). Previous studies have shown significant improvements in crop yield as well as crop water and nutrient use efficiency in plants cultivated under IAAS (Dey et al. 2010; Mariscal-Lagarda et al. 2012; M.Moursy et al. 2019; Farrag et al. 2021; Sewilam et al. 2022). Likewise, several studies have also shown improved water quality, survival, and growth performance of fish reared under IAAS (Mariscal-Lagarda et al. 2012; Farrag et al. 2021; Sewilam et al. 2022). For successful implementation and operation of the IAAS especially under saline conditions, the selection of suitable fish and crops of high economic value is paramount.
Stripped catfish (Pangasianodon hypophthalmus) is a highly tolerant freshwater fish to various salinity ranges and thus can be considered a potential candidate species for inland saline aquaculture (Kumar et al. 2017; Hossain et al. 2021). Its high market value is attributed to its palatability, high protein content, lower number of spines, rapid growth rate, easy acceptance of artificial feed, and ability to tolerate environmental stress (high temperature, crowding, and salinity) (Ahmed et al. 2010; Singh and Lakra 2012; Kumar et al. 2017; Jahan et al. 2019). Previous studies have shown that rearing P. hypophthalmus at high water salinities reaching 10,000 to 15,000 ppm does not negatively impact the growth performance and the general well-being of fish (Nguyen et al. 2014; Kumar et al. 2017; Hieu et al. 2021). Likewise, previous studies have also shown that P. hypophthalmus can tolerate crowding stress (Nageswari et al. 2022b, a), high-temperature stress (28–32 °C) (Shahjahan et al. 2018; Islam et al. 2019), and poor water quality (Okomoda et al. 2019; Hasibuan et al. 2021). However, P. hypophthalmus wastewater (effluents) is highly rich in nutrients and its poor disposal could lead to environmental pollution (De Silva et al. 2010; Anka et al. 2014; Nhut et al. 2019; Meena et al. 2022). Therefore, the utilization of P. hypophthalmus wastewater as a water and nutrient source for plants could hinder environmental pollution and improve plant growth and yields.
Sorghum (Sorghum bicolor (L.) Moench) is one of the most cultivated foods and fodder crops in Africa with the potential to improve food and feed security in drought-stricken, water-scarce, and saline regions (Bavei et al. 2011; Hadebe et al. 2017; Mansour et al. 2021; Amombo et al. 2022). This is attributed to its drought tolerance, moderate tolerance to salinity, as well as the ability to grow in poor nutrient soils (Hufnagel et al. 2014; Mansour et al. 2021; Abreha et al. 2022). Previous studies have shown that cultivation of sorghum under moderately saline conditions does not significantly impact its growth, grain or forage yield, and forage quality (Francois et al. 1984; Hedayati-Firoozabadi et al. 2020; Gois et al. 2022). To the best of our knowledge, no studies have so far been conducted on the integration of P. hypophthalmus aquaculture and S. bicolor production in IAAS under brackish water conditions. The current study, therefore, aims to (1) assess the effect of saline aquaculture wastewater on the growth, yield, forage quality, and nutritive composition of S. bicolor grains under an IAAS and (2) assess the effect of different water qualities on fish survival, growth performance, and health status.
Material and methods
Plant material and experimental design
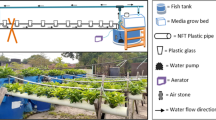
Sorghum seeds were obtained from the Agricultural Research Center (ARC) in Giza, Egypt. A field experiment was conducted between August and November 2021 at the Center for Applied Research on the Environment and Sustainability (CARES), the American University in Egypt, New Cairo, Egypt (30° 01′ 11.7″ N31° 29′ 59.8″ E). The experiment was conducted in a randomized completely block design of four salinity treatments with three replicates, i.e., control (freshwater mixed with inorganic fertilizers), 5000 ppm, 10,000 ppm, and 15,000 ppm (Fig. 1). Table 1 shows the chemical properties of the salt used in this study (Mugwanya et al. 2023). The soil's physical and chemical properties are presented in Table 2. Within the growing season, the maximum average temperature was 27.5 °C, average relative humidity was 62%, solar radiation was 207.58 Wm2, and wind speed was 1.56 m s−1.
Crop cultivation
Seeds were hand-sewn in rows with inter and intra-row spacing of 30 cm and 50 cm respectively based on a planting density of 66,667 plants ha−1. Weeding was done by hand and plants were drip irrigated according to crop water requirements. The crop water requirements were estimated using the Food and Agriculture Organization (FAO)-CROPWAT model (Gabr 2022). As such, water was pumped from the tanks (i.e., aquaculture units and the control) to the designated grow beds by an automated drip irrigation system to irrigate the plants according to the set conditions. Pest and insect control was conducted according to the recommendations of the Egyptian Ministry of Agriculture. Likewise, fertigation (control treatment) for sorghum was performed according to the recommendations of the Egyptian Ministry of Agriculture.
Sampling and agronomical trait measurements
Six plants within the borders per replicate were randomly tagged for agronomical trait measurements. At each data collection time point, plant heights were measured from the crown to the terminal growing point of the plant using a meter rule. Stalk diameters were measured from the second internode from the bottom-up of the plant using digital vernier calipers and averages were determined. The number of internodes and leaf number per plant was obtained by counting and averages were determined. The leaf area was calculated as shown below according to the equation by Elsahookie and Cheyed (2014).
where L and W are the leaf length and leaf width, respectively.
Chlorophyll content was measured in the early morning at each data collection time point using an MC-100 chlorophyll meter from Apogee Instruments, Inc., Utah, USA, and data was expressed as soil–plant analysis development (SPAD) averages.
For the determination of fresh weights, six plants per replicate were divided into stalks, leaves, and panicles. Stalks were cut into smaller pieces of approximately 5 cm. All plant fractions were weighed to obtain the fresh weights and then oven-dried at a constant weight at 70 °C for 3 days to obtain the dry weights, and the data were expressed as grams per plant.
Seed nutrient composition analysis
For each treatment, a 300-mg sample of seeds was obtained for microwave digestion (Model: speed wave Entry DAP-60 K). Briefly, the seeds were placed in a digestion vessel and 65% 3.0 ml of nitric acid (HNO3) and 35% hydrogen peroxide (H2O2) were added. The mixture was carefully stirred using a clean glass rod and gently shaken. The vessel was closed 10 min after shaking and the sample was heated in the microwave. After cooling, the clear solution was obtained and used for elemental analysis (phosphorus, calcium, magnesium, zinc, copper, manganese, and iron) using inductively coupled plasma–optical emission spectroscopy (ICP-OES, Model: Ultima 2 JY Plasma). All measurements were performed using an Agilent 4210 MP-AES fitted with a double-pass cyclonic spray chamber and a OneNeb Series 2 nebulizer. Nitrogen was supplied using an Agilent 4107 Nitrogen Generator. All wavelengths were selected from the MP Expert software library, according to the sensitivity that was required.
Fiber fraction, nutrient composition, and in vitro digestibility of leaves and stalks
Fiber fraction analysis is a vital technique used for the determination of the forage quality of animal feeds. It takes into account the following parameters: neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL). In this study, samples per replicate in each main treatment were pooled and taken to the Regional Center for Food and Feed, Giza, Egypt. Briefly, stalks and leaves were separately ground using a Wiley mill and the fine powder passed through a 1-mm screen. Neutral detergent fiber (NDF, AOAC no. 2002.04), acid detergent fiber (ADF, AOAC no. 973.18), and acid detergent lignin (ADL, AOAC no. 973.18) were sequentially determined by semiautomatic ANKOM220 Fiber Analyzer (ANKOM Technology, Macedon, NY, USA). Cellulose (ADF-ADL), hemicellulose (NDF-ADF), and lignin were calculated from the organic matter of the detergent fiber fractions respectively. The total nitrogen content of the samples was determined by the Kjeldahl technique followed by the determination of concentrations of crude protein (CP) according to the Association of Official Analytical Chemists 2016 (AOAC no.984.13 and no. 968.06, respectively). Fat and fiber contents were determined according to the approved methods given in AOAC (1984).
Assessment of the quality of animal feeds has for a long time been performed by in vitro digestibility and gas production techniques. This technique involves the use of Rumen fluid extracted from animals as an inoculum to mimic the in vivo fermentation of feed, thus allowing a proper estimation of the nutritive composition and fermentation kinetics of ruminant feeds through gas production. Merits of this technique over the in vivo fermentation in the determination of the nutritive value of feedstuffs include the following: It is cheaper, faster, less labor intensive, and suitable for both small quantities of feed and large-scale evaluation of ruminant feeds (Getachew et al. 1999). In this study, the gas production technique was performed according to Menke and Steingass (1988) at the Regional Center for Food and Feed, Agricultural Research Center, Giza. Briefly, ammonium-free rumen fluid was collected in equal proportions from two animal donors (sheep) before their morning feed and put into thermo flasks. The rumen fluid was later filtered through a 1-mm sieve and the obtained filtrate was incubated at 39 °C. Rumen 27 liquor and buffer solution were mixed in a ratio of 1:2 (v/v), and all laboratory procedures for handling rumen liquor were conducted under a continuous flow of carbon dioxide gas. Two hundred-milligram test samples were fed into 100-ml capacity graduated plastic syringes, and the lubricated pistons were inserted into the syringes. Thirty milliliters of rumen liquor (inoculum) was introduced into the plastic syringes via silicon tubes at the tips of the syringes, and these were subjected to incubation (± 39 °C). Gas production was measured at 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 h. This experiment was conducted in triplicates. In vitro true digestible dry matter (IVTD), digestible organic matter (DOM), metabolic energy (ME), and net energy (NE) were calculated as described by Menke and Steingass (1988). Total digestible nutrients (TDN) were calculated from ME values as per the equation of NRC (1989). Microbial protein (MP) was calculated as described by Czerkawski (1986), whereas short-chain fatty acids (SCFA) were calculated as described by Getachew et al. (1999).
Fish growth performance, hematological, and serum biochemical parameters
Stripped catfish (Pangasianodon hypophthalmus, initial weight 6.0 g) were obtained from a local distributor and stocked in each tank at a stocking density of 100 fish/900 l. The fish were fed two to three times daily with commercial pellets supplied by Skretting Egypt. The pellets contained 28% crude protein, 5% crude lipid, 6% crude fiber, 13% ash, and 9% moisture. The feeding pattern and frequency were according to the fish biomass percentage of 2–3% depending on the growth and satiation. Fish growth parameters such as feed conversion ratio (FCR), specific growth rate (SGR), feed intake (FI), body weight gain (BWG), survival rate (SR%), and condition factor (CF) were calculated according to the formula below.
For hematological parameters, a group of three fish per treatment was randomly collected and a 1-ml sterile syringe was used to draw blood from the mid-ventral line behind the anal fin and collected in purple top EDTA blood collection tubes and immediately sent to the lab for complete blood count (CBC) test. Of blood per treatment, 150 µl was used for determining the CBC using the human automatic hematology analyzer (XP-300, Sysmex Corporation).
Serum was obtained by centrifuging blood at 1000 × g for 3 min and used for the analysis of serum biochemical parameters. Briefly, 1 ml of serum was obtained and used for quantification of total protein, albumin, blood urea nitrogen (BUN), and creatinine using the Total Protein Biuret Reagent kit (Cat. No. 310 001), Albumin-BCG kit (Cat. No. 211 001), UREA/BUN-Liquizyme kit (Cat No. 318 002), and Creatinine-Jaffe kit (Cat. No. 234 001) from SPECTRUM, respectively. Globulin was calculated as Total protein − Albumin. Absorbance at different wavelengths was read using the BIOMED DIAGNOSTICS (Model: CLINI-CHEM) serum analyzer.
Fish wastewater quality
Fish wastewater quality parameters such as water temperature, pH, and dissolved oxygen (DO) were closely monitored using automated digital Nilebot technologies by Conative Labs to fit the ideal required levels for catfish. Likewise, water quality parameters such as ammonia, ammonium, ammonia–nitrogen, nitrite–nitrogen, and nitrate–nitrogen were quantified once every 2 weeks until the end of the growing season using water analysis chemical kits from Hanna instruments. Briefly, 100 ml of water was collected from the tanks before feeding and immediately taken to the lab for analysis of ammonia, nitrite, and nitrate concentrations using a photometer along with the ammonia reagent kit (H193715-01), nitrite reagent kit (H193707-01), and nitrate reagent kit (H193728-01) from HANNA instruments. Specific absorbance of the nitrogenous elements was measured using the Aquaculture Photometer device (H183303). The device was set to display the concentrations of ammonia–nitrogen, ammonium, nitrate–nitrogen, and nitrite–nitrogen in milligrams per liter. Real-time analysis results were presented in the manuscript as mean values read from the photometer.
Statistical analysis
Data sets were tested for normality before analysis of variance (ANOVA) was conducted. SPSS software (version 22) was used to carry out both one-way and two-way ANOVAs to test for significant differences (p < 0.05) between the treatments. Tukey’s HSD (honestly significant difference) test was used to compare differences among the treatment means when significant F values were observed at p < 0.05 level. Principal component analysis summarizing the variations in micro- and macronutrient compositions of sorghum seeds as well as correlograms showing correlation analysis of several parameters were constructed using the “prcomp” function and corrgram package of R Statistical Programming Language (version 4.1.0), respectively.
Results
Agronomical trait parameters
Table 3 summarizes data on the agronomical trait parameters of sorghum at different data collection time points. Results on plant height indicated no significant differences among all salinity treatments and the control at 30, 60, and 90 days after sowing (DAS). Plant heights increased with increasing plant maturity with 60 and 90 DAS significantly recording higher plant heights compared to 30 DAS. No significant interaction was noted between DAS × plant heights.
For stalk diameter, no significant differences were noted among all the salinity treatments and the control. However, stalk diameter increased with increasing plant maturity with 60 and 90 DAS significantly recording higher values for stalk diameter compared to 30 DAS. No significant interaction was noted between DAS × stalk diameter.
Results on the number of internodes showed that the control significantly recorded higher values for the number of internodes per plant compared to 5000, 10,000, and 15,000 ppm at 60 and 90 DAS. Overall, 90 DAS significantly recorded the highest values for the number of internodes per plant followed by 60 and 30 DAS, respectively. A significant interaction between DAS × number of internodes was noted (p < 0.05).
Results of leaf number indicated no significant differences in the average leaf number per plant among all salinity treatments and the control at 30 and 60 DAS. However, the average leaf number per plant of the control significantly increased (p < 0.05) by 39.98% compared to 5000, 10,000, and 15,000 ppm salinity treatments at 90 DAS. Overall, 60 DAS significantly recorded higher values for leaf number per plant compared to 30 and 90 DAS. A significant interaction was noted between DAS × leaf number per plant (p < 0.0001). For leaf area, no significant differences in the average leaf area were noted among the salinity treatments and the control at 60 and 90 DAS. Overall, 60 DAS significantly recorded higher values for the average leaf area compared to 30 and 90 DAS. No significant interaction was noted between DAS × leaf area.
Data on the leaf chlorophyll content (SPAD) indicated no significant differences among all salinity treatments and the control across all the data collection time points. However, 30 DAS significantly recorded higher SPAD values compared to 60 and 90 DAS. No significant interaction was noted between DAS × SPAD.
Forage and grain yield
Results of the forage and grain yield are presented in Table 4. The control treatment recorded the highest forage fresh and dry yield compared to other treatments. However, no significant differences in yield were noted among all the treatments. Likewise, no significant differences in grain yield were noted among all the treatments.
Correlation between yield and the average plant growth parameters of Sorghum bicolor cultivated under different salinity treatments
Figure 2 shows the results of the Pearson’s correlation analysis between yield and the average growth parameters of sorghum cultivated under different salinity treatments in the IAAS. A strong correlation was observed between the grain yield, forage fresh yield, forage dry yield, internode number, and leaf number. Plant heights were shown to be moderately correlated to SPAD, internode number, leaf number, grain yield, forage fresh yield, and forage dry yield. Likewise, SPAD was shown to be moderately correlated to grain yield, forage fresh yield, forage dry yield, internode number, and leaf number. Stalk diameter and leaf area were shown to be negatively correlated to all other plant growth parameters and yield.
A correlogram showing correlation analysis of yield and the average growth parameters of sorghum. Blue and brown colors are positive and negative significant correlations, respectively, according to Pearson’s correlation analysis. The color intensity and circle size are proportional to the correlation coefficient
Seed nutrient composition and principal component and correlation analysis on nutrient composition of sorghum seeds
The results of the seed nutrient composition analysis are presented in Table 5. Data indicate no significant differences in the nutrient composition of macro elements (phosphorus, calcium, and magnesium) among all salinity treatments and the control. For microelement composition, 15,000 ppm had the highest zinc, copper, manganese, and iron content compared to other salinity treatments and the control, but no significant differences were noted.
Results of the correlation matrix analysis (Fig. 3) showed a strong and positive correlation between copper (Cu) and zinc (Zn) (0.875), manganese (Mn) and Zn (0.825), as well as iron (Fe) and zinc (0.727). Likewise, a strong and positive correlation was noted between Mn and Cu (0.841), Fe and Cu (0.836), as well as Mn and Fe (0.888). Overall, macronutrients especially calcium (Ca) and magnesium (Mg) showed a moderate and positive correlation with micronutrients.
Figure 4 summarizes the results of the principal component analysis. Phosphorus contributed less to the variation of the principal components (i.e., Dim 1 and Dim 2), whereas other elements contributed more to the variation of the principal components.
Fiber fraction and nutrient composition of leaves and stalks
Table 6 summarizes the results on fiber fraction and nutrient composition of leaves and stalks. Fiber fraction analysis indicated no significant difference in the neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) of leaves and stalks among all the treatments. Likewise, no significant differences were noted in hemicellulose (HEM), cellulose (CEL), and lignin (LIG) content of leaves and stalks among all the treatments. The crude protein (CP) content in the leaves of the control treatment was significantly higher (p < 0.05) compared to that of other treatments. No significant differences in the leaves fat and fiber content were noted among all the treatments. For stalks, no significant differences were noted in the protein and fat content among all the treatments. However, the salinity treatment of 10,000 ppm significantly had a higher fiber content (p < 0.05) in stalks compared to the control. Overall, the cellulose content of leaves was lower (20–28%) compared to that of the stalks (30–32%). However, the CP of leaves was higher (5–9%) compared to that of stalks (2–3%).
In vitro digestibility of leaves and stalks
Results on the in vitro digestibility of leaves and stalks are presented in Table 7. No significant differences were noted in the in vitro true digestibility of dry matter (IVTD), digestible organic matter (DOM), metabolic energy (ME), short-chain fatty acids (SCFA), total digestible nutrients (TDN), net energy (NE), and microbial protein (MP) in the leaves and stalks among all salinity treatments and control.
Correlation analysis between fiber fraction, nutrient composition, and in vitro digestibility of leaves and stalks
Figure 5 shows results of the Pearson’s correlation analysis between fiber fraction, nutrient composition, and in vitro digestibility of leaves and stalks of sorghum cultivated under different treatments in the IAAS. For the leaves (Fig. 5a), there was a significantly strong and positive correlation between NDF and ADF. Likewise, there was a strong and positive correlation between ADL, HEM, and CEL. A significantly strong and positive correlation was also noted between the fiber content and NDF, ADF, ADF, and fat content. IVTD was positively correlated with NE, DOM, MP, ME, and TDN. For stalks (Fig. 5b), there was a significant and positive correlation between ADF and CEL. Likewise, a significant positive correlation was noted between ADL, NDF, and HEM. The fiber content strongly and significantly correlated with ADL, NDF, and HEM. ME strongly and significantly correlated with DOM, MP, SCFA, TDN, and NE.
A correlogram showing correlation analysis of fiber fraction, nutrient composition, and in vitro digestibility in (a) leaves and (b) stalks of sorghum. Blue and brown colors are positive and negative significant correlations, respectively, according to Pearson’s correlation analysis. The color intensity and circle size are proportional to the correlation coefficient. LIG, lignin; HEM, hemicellulose; CEL, cellulose; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; IVTD, in vitro true digestible dry matter; DOM, digestible organic matter; ME, metabolic energy; SCFA, short-chain fatty acids; NE, net energy; TDN, total digestible nutrients
Fish growth performance
Table 8 summarizes the results of the growth performance of Pangasianodon hypophthalmus reared under different salinity treatments. No significant differences in the final weight were observed among all the salinity treatments. However, data on the body weight gain (BWG) indicated that 15,000 ppm salinity treatment significantly recorded lower values for the BWG compared to 10,000 ppm and 5000 ppm salinity treatments (p < 0.05). Likewise, the 15,000 ppm treatment significantly (p < 0.05) recorded the highest values for the feed conversion ratio (FCR) compared to 10,000 ppm and 5000 ppm salinity treatments. Data on the specific growth rate (SGR) indicated that fish reared under the 5000 and 10,000 ppm salinity treatments significantly (p < 0.05) had higher SGR compared to the 15,000 ppm treatment (p < 0.05). For the condition factor (CF), fish reared under 5000 ppm significantly (p < 0.05) recorded higher values for CF compared to those reared under 15,000 ppm treatment. No significant differences in the survival rate were noted among all the salinity treatments.
Hematological and serum biochemical parameters
The results of the hematological parameters are presented in Fig. 6. No significant differences in the concentration of white blood cells (Fig. 6a), red blood cells (Fig. 6b), hemoglobin (Fig. 6c), hematocrit (Fig. 6d), mean corpuscular volume (Fig. 6e), and mean corpuscular hemoglobin (Fig. 6f) were noted across all salinity treatments.
Hematological parameters of fish reared under different salinity treatments. a White blood cells (WBC), b red blood cells (RBC), c hemoglobin (HGB), d hematocrit (HCT), e mean corpuscular volume (MCV), f mean corpuscular hemoglobin (MCH). Data presented as mean ± SD (n = 3). Error bars represent standard deviation. Bar columns having different letters are significantly different (p < 0.05)
Data on the serum biochemical parameters are presented in Fig. 7. Results indicated that 5000 ppm salinity treatment significantly (p < 0.05) recorded higher values for serum total protein concentration compared to 15,000 ppm treatment (Fig. 7a). No significant differences in albumin concentration were noted among all the salinity treatments (Fig. 7b). However, the 5000 ppm salinity treatment significantly (p < 0.05) recorded higher values for globulin concentration compared to the 15,000 ppm treatment (Fig. 7c). For blood urea nitrogen (BUN), the results showed that 15,000 ppm salinity treatment significantly (p < 0.05) recorded higher values for BUN compared to 10,000 ppm treatment (Fig. 7d). No significant differences in creatinine concentration were noted among all salinity treatments (Fig. 7e).
Serum biochemical parameters of fish reared under different salinity treatments. a Total protein, b albumin, c globulin, d blood urea nitrogen (BUN), and e creatinine. Data presented as mean ± SD (n = 3). Error bars represent standard deviation. Bar columns having different letters are significantly different (p < 0.05)
Fish wastewater quality
The fish wastewater quality parameters are presented in Fig. 8. Although the 5000-ppm salinity treatment recorded the lowest values for ammonia, ammonium, ammonia–nitrogen, nitrite–nitrogen, and nitrate nitrogen, no significant differences were noted with other salinity treatments.
Water quality parameters of fish wastewater under different salinity treatments. a NH3 (ammonia); b NH4+ (ammonium); c NH3–N (ammonia–nitrogen); d NO2 − –N (nitrite–nitrogen); e NO3 − –N (nitrate–nitrogen). Data expressed as mean ± SE. Error bars represent the standard error. Different lower superscript letters within each column indicate a significant difference within treatments (p < 0.05)
Discussion
The reuse of aquaculture wastewater in agriculture is becoming more prominent in arid and semi-arid regions of the world due to water scarcity (Gengmao et al. 2010; El-kady and Suloma 2013; Chen et al. 2017; Calone et al. 2020). Aquaculture wastewater is rich in organic matter and nutrients such as nitrogen (N), phosphorus (P), and potassium (K) which are important for plant growth (Omotade et al. 2019; Kimera et al. 2023; Terkula et al. 2023). In our study, irrigating sorghum with saline aquaculture wastewater did not show significant detrimental effects in the tested agro-morphological parameters (plant height, leaf area, stalk diameter, and SPAD) and hence in agreement with previous studies (Guimarães et al. 2019, 2022; Kolozsvári et al. 2022). This is because sorghum is moderately tolerant to soil and water salinity of up to 5440 and 2700 ppm respectively above which a 16% reduction in yield is expected (Calone et al. 2020). For the number of internodes per plant, it was observed that the three salinity treatments significantly decreased the internode number compared to the control. This is because the accumulation of sodium ions in plant tissues is toxic, and it affects cell division and expansion (Alizadeh et al. 2011). On the other hand, however, higher water salinities (i.e., 5000 ppm, 10,000 ppm, and 15,000 ppm) did not show significant differences in both forage and grain yield with the control treatment. This could be attributed to several factors such as the presence of plant growth-promoting bacteria (PGPBs) in aquaculture wastewater and the crop variety cultivated. Previous literature indicates that PGPBs not only promote plant growth but also act as elicitors of salinity tolerance in plants (Vacheron et al. 2013; Daliakopoulos et al. 2016; Kumar et al. 2020). Furthermore, an increase in hemicellulose content in leaves and stalks of sorghum was noted especially under highly saline conditions (10,000 ppm and 15,000 ppm) which we anticipate could have led to improved salinity tolerance in sorghum. Tissue-specific up- and down-regulations of the expression of cell wall remodeling enzymes such as xyloglucan endotransglucosylase/hydrolases (XTHs) responsible for hemicellulose biosynthesis have previously been linked to positively or negatively regulate salinity tolerance in several plant species such as Salicornia europaea (Tiika et al. 2021), Medicago truncatula (Xuan et al. 2016), Vitis vinifera L. (Qiao et al. 2022), and Populus sp. (Cheng et al. 2021).
Pearson’s correlation coefficient analysis indicated positive correlations between SPAD (leaf chlorophyll content) and yield parameters (i.e., grain yield, fresh and dry forage yield). Chlorophyll has a direct role in photosynthesis and thus has an impact on the photosynthetic capacity, growth, and yield of crops (Liu et al. 2019; Song et al. 2019; Wasaya et al. 2021; Zhou et al. 2022). In our study, lower forage and grain yields, although non-significant, were noted in highly saline treatment conditions compared to the control, and this correlated with a decrease in SPAD values. Salinity not only disintegrates the chlorophyll structure and integrity but also denatures enzymes responsible for chlorophyll biosynthesis, hence leading to a decrease in chlorophyll content of leaves, growth, and yield (Mugwanya et al. 2022; Nawaz et al. 2022). Similar results have previously been reported in Oryza sativa L. (Hakim et al. 2014; Kumar et al. 2021), Triticum aestivum (Eroglu et al. 2020; Adil et al. 2022), Hordeum vulgare L. (Noreen et al. 2021; Alharbi et al. 2022), and Zea mays (Bouras et al. 2021; Ali et al. 2022).
The current study also indicated variations in seed macro- and micronutrient contents in response to different salinity treatments and the control. High accumulation of micronutrients such as zinc (Zn), copper (Cu), manganese (Mn), and iron (Fe) in seeds of sorghum plants cultivated under high salinity (15,000 ppm) is in agreement with previous studies on Triticum aestivum (Faran et al. 2019), Cucumis sativus L. (Mugwanya et al. 2022), Hordeum vulgare L. (Ali et al. 2012), and Gossypium hirsutum L. (Dai et al. 2014). Micronutrients such as Zn, Cu, and Fe have been linked to play a significant role in antioxidant enzyme activity under plant abiotic stress (Bian and Jiang 2009; Babaei et al. 2017; Hassan et al. 2020). In the same regard, increased accumulation of calcium (Ca) in seeds of sorghum plants cultivated under high salinity (15,000 ppm) indicates the role of Ca in modulating abiotic stress. For instance, calcium-dependent protein kinases (CDPKs) have been reported to modulate abiotic stress through activation and regulation of several enzymes, genes, transcription factors, and ion channels, hence supporting plant adaptation to different abiotic stress factors (Franz et al. 2011; Atif et al. 2019; Aslam et al. 2021).
The forage quality in terms of fiber fraction and nutrient composition of the sorghum stover (leaves and stalks) was assessed. Generally, stalks had higher NDF, ADF, and ADL contents compared to the leaves and this is in agreement with previous studies (Kaplan et al. 2014; Lamidi and Konyeha 2020; Wahyono et al. 2021). Moreover, the fiber content in stalks was higher in plants irrigated with saline aquaculture wastewater compared to the control. Fiber is majorly composed of cellulose and lignin (Yang et al. 2019). However, it has been previously reported that the lignification of plant tissues increases with an increase in salinity stress, but this is species-dependent (Zou et al. 2021; Wang et al. 2023). Except for the control treatment, the crude protein (CP) content of leaves and stalks of sorghum was below the recommended range (7%) for good-quality forage (Milford and Minson 1966) in all the salinity treatments. This could be attributed to a higher concentration of N in the chemical fertilizers used in the control treatment relative to that in saline aquaculture wastewater. Previous studies have also shown that the application of N fertilizers in sorghum improves their forage quality in terms of crude protein content (Ates et al. 2019; Holman et al. 2019; Sriagtula et al. 2019). Rumen fermentation represented as in vitro true digestible dry matter (IVTD), digestible organic matter (DOM), metabolic energy (ME), net energy (NE), and short-chain fatty acids (SCFA) was assessed in both leaves and stalks. Lower values for IVTD, DOM, ME, NE, and SCFA were recorded in contrast to recent studies on brown midrib mutant (BMR) sorghum (Wahyono et al. 2019, 2023; Umesh et al. 2022) and BMR millet (Oskey et al. 2023). The difference in results could be attributed to lower lignin and fiber contents in BMR forage crops compared to non-BMR forage and grain crops. This is because lignin, a complex phenolic polymer deposited in the secondary cell wall of plants, slows down the enzymatic breakdown of complex sugars such as cellulose, hence decreasing the forage digestibility in ruminants (Saluja et al. 2021). Moreover, a meta-analysis of dairy cows fed conventional sorghum or corn silages compared with BMR silage sorghum indicated improved milk production, increased milk fat concentration, and lactose yield in cows fed on BMR silage sorghum compared to those fed conventional sorghum or corn silage (Sánchez-Duarte et al. 2019).
Stripped catfish (Pangasianodon hypophthalmus) were reared under different water salinities (i.e., 5000 ppm, 10,000 ppm, and 15,000 ppm), and results indicated lower values for final weight, body weight gain (BWG), and specific growth rate (SGR) in fish reared at 15,000 ppm compared to those reared at 5000 and 10,000 ppm. This could be attributed to the lower feed intake of fish due to salinity stress which resulted in poor feed conversion ratio (FCR) and hence in agreement with previous studies (Nguyen et al. 2014; Meritha et al. 2018; Ha et al. 2021; Hieu et al. 2021). P. hypophthalmus is a freshwater fish species whose osmoregulation is disrupted when exposed to water salinity levels exceeding 10,000 ppm (Nguyen et al. 2014). Moreover, the disruption of osmoregulation negatively impacted the health status of fish as indicated by a decline in values for hematological parameters (red blood cells, white blood cells, hemoglobin, and hematocrit; Fig. 6), serum biochemical parameters (total protein, globulin, and creatinine; Fig. 7), and water quality (i.e., accumulation of nitrogenous compounds in water; Fig. 8).
Conclusion
According to our study’s findings, irrigating grain sorghum with aquaculture wastewater of varying salinity levels (i.e., 5000 ppm, 10,000 ppm, and 15,000 ppm) leads to the accumulation of toxic ions in plant tissues which negatively impacts certain plant growth parameters most notably the internode number. However, these salinity treatments do not lead to significant reductions in forage and grain yield production. Likewise, these salinity levels do not significantly reduce the nutritive composition of sorghum seeds as well as the forage quality of leaves and stalks in terms of fiber fraction, nutritive composition, and in vitro digestibility. Although rearing striped catfish (Pangasianodon hypophthalmus) at salinity levels above 10,000 ppm negatively impacts its growth performance, this fish species can still survive at water salinities reaching up to 15,000 ppm. Overall, the integration of sorghum production and striped catfish production under salinity levels of 5000 ppm could enhance economic returns to farmers regarding increased grain, forage, and fish yield in conditions of freshwater scarcity.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
A.O.A.C (1984) Official Methods of Analysis. Association of Official Analytical Chemists. 14th Edition, AOAC, Arlington
Abd El-Gawad A, Morsy A (2017) Integrated impact of organic and inorganic fertilizers on growth, yield of maize (Zea mays L.) and soil properties under Upper Egypt conditions. J Plant Prod 8:1103–1112. https://doi.org/10.21608/jpp.2017.41121
Abreha KB, Enyew M, Carlsson AS et al (2022) Sorghum in dryland: morphological, physiological, and molecular responses of sorghum under drought stress. Planta 255:1–23. https://doi.org/10.1007/s00425-021-03799-7
Adil M, Bashir S, Bashir S et al (2022) Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front Plant Sci:1–9. https://doi.org/10.3389/fpls.2022.932861
Ahmed N, Alam MF, Hasan MR (2010) The economics of sutchi catfish (Pangasianodon hypophthalmus) aquaculture under three different farming systems in rural Bangladesh. Aquac Res 41:1668–1682. https://doi.org/10.1111/j.1365-2109.2010.02549.x
Alharbi K, Rashwan E, Mohamed HH et al (2022) Application of silica nanoparticles in combination with two bacterial strains improves the growth, antioxidant capacity and production of barley irrigated with saline water in salt-affected soil. Plants 11:2026
Ali AYA, Ibrahim MEH, Zhou G et al (2022) Interactive impacts of soil salinity and jasmonic acid and humic acid on growth parameters, forage yield and photosynthesis parameters of sorghum plants. S Afr J Bot 146:293–303. https://doi.org/10.1016/j.sajb.2021.10.027
Ali S, Cai S, Zeng F et al (2012) Effect of salinity and hexavalent chromium stresses on uptake and accumulation of mineral elements in barley genotypes differing in salt tolerance. J Plant Nutr 35:827–839. https://doi.org/10.1080/01904167.2012.663438
Alizadeh MR, Dabbaghi A, Rahimi-Ajdadi F et al (2011) Effect of salinity and irrigation regimes on the internode physical variations of rice stem. Aust J Crop Sci 5:1595–1602
Amombo E, Ashilenje D, Hirich A et al (2022) Exploring the correlation between salt tolerance and yield: research advances and perspectives for salt-tolerant forage sorghum selection and genetic improvement. Planta 255:1–16. https://doi.org/10.1007/s00425-022-03847-w
Anka I, Faruk M, Hasan M, Azad M (2014) Environmental issues of emerging pangas (Pangasianodon hypophthalmus) farming in Bangladesh. Progress Agric 24:159–170. https://doi.org/10.3329/pa.v24i1-2.19118
Arnell NW (2018) Climate change and global water. J Diabetes Investig 43
Aslam S, Gul N, Mir MA et al (2021) Role of jasmonates, calcium, and glutathione in plants to combat abiotic stresses through precise signaling cascade. Front Plant Sci 12:1–29. https://doi.org/10.3389/fpls.2021.668029
Ates E, Tenikecier HS, Faculty A (2019) Hydrocyanic acid content, forage yield and some quality features of two Sorghum-Sudan grass hybrid cultivars under different nitrogen doses in Thrace, Turkey. Ciurrent Trends Nat Sci 8:55–62
Atif RM, Shahid L, Waqas M et al (2019) Insights on calcium-dependent protein kinases (CPKs) signaling for abiotic stress tolerance in plants. Int J Mol Sci 20:5298. https://doi.org/10.3390/ijms20215298
Awaad MS, Ahmed AR, Instit ER (2010) The negative role of soil salinity and waterlogging on crop productivity in the north- eastern region of the Nile Delta, Egypt. Res J Agric Biol Sci 6:378–385
Babaei K, Sharifi RS, Pirzad A, Khalilzadeh R (2017) Effects of bio fertilizer and nano Zn-Fe oxide on physiological traits, antioxidant enzymes activity and yield of wheat (Triticum aestivum L.) under salinity stress. J Plant Interact 12:381–389. https://doi.org/10.1080/17429145.2017.1371798
Bavei V, Shiran B, Arzani A (2011) Evaluation of salinity tolerance in sorghum (Sorghum bicolor L.) using ion accumulation, proline and peroxidase criteria. Plant Growth Regul 64:275–285. https://doi.org/10.1007/s10725-011-9568-z
Bian S, Jiang Y (2009) Scientia Horticulturae Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci Hortic (Amsterdam) 120:264–270. https://doi.org/10.1016/j.scienta.2008.10.014
Bouras H, Bouaziz A, Choukr-allah R et al (2021) Phosphorus fertilization enhances productivity of forage corn (Zea mays L.) irrigated with saline water. Plants 10:2608
Calone R, Sanoubar R, Lambertini C et al (2020) Salt tolerance and Na allocation in Sorghum bicolor under variable soil and water Salinity. Plants 9:561
Chen L, Feng Q, Li C et al (2017) Impacts of aquaculture wastewater irrigation on soil microbial functional diversity and community structure in arid regions. Sci Rep:1–10. https://doi.org/10.1038/s41598-017-11678-z
Cheng Z, Zhang X, Yao W et al (2021) Genome-wide identification and expression analysis of the xyloglucan endotransglucosylase/hydrolase gene family in poplar. BMC Genomics 22:804
Czerkawski JW (1986) An intriduction to rumen studies. Oxford: Pergamon Press
Dai JL, Duan LS, Dong HZ et al (2014) Improved nutrient uptake enhances cotton growth and salinity tolerance in saline media. J Plant Nutr 37:1269–1286. https://doi.org/10.1080/01904167.2014.881869
Daliakopoulos IN, Tsanis IK, Koutroulis A et al (2016) The threat of soil salinity: a European scale review. Sci Total Environ 573:727–739. https://doi.org/10.1016/j.scitotenv.2016.08.177
De Silva SS, Ingram BA, Nguyen PT et al (2010) Estimation of nitrogen and phosphorus in effluent from the striped catfish farming sector in the Mekong Delta, Vietnam. Ambio 39:504–514. https://doi.org/10.1007/s13280-010-0072-x
Dey MM, Paraguas FJ, Kambewa P, Pemsl DE (2010) The impact of integrated aquaculture-agriculture on small-scale farms in Southern Malawi. Agric Econ 41:67–79. https://doi.org/10.1111/j.1574-0862.2009.00426.x
Ding Z, Koriem MA, Ibrahim SM et al (2020) Seawater intrusion impacts on groundwater and soil quality in the northern part of the Nile Delta, Egypt. Environ Earth Sci 79:1–11. https://doi.org/10.1007/s12665-020-09069-1
El-kady AFY, Suloma A (2013) Towards wastewater-aquaculture-agriculture integration in arid and semi-arid regions: utilization of aquaculture effluents in the irrigation of Khaya and Mahogany seedlings. J Hortic Sci Ornam Plants 5:227–237. https://doi.org/10.5829/idosi.jhsop.2013.5.3.1130
El Demerdash D, Nour MMED, El-badry H et al (2022) Effectiveness of adding a salt tolerant crop to the Egyptian crop pattern to adapt with the water salinity and shortage conditions. Am Acad Sci Res J Eng Technol Sci 861:202–216
Elsahookie MM, Cheyed SH (2014) Estimating sorghum leaf area by measuring one leaf length. Iraqi J Agric Sci 45:1–5
Eroglu CG, Cabral C, Ravnskov S et al (2020) Arbuscular mycorrhiza influences carbon-use efficiency and grain yield of wheat grown under pre- and post-anthesis salinity stress. Plant Biol 22:863–871. https://doi.org/10.1111/plb.13123
Faran M, Farooq M, Rehman A et al (2019) High intrinsic seed Zn concentration improves abiotic stress tolerance in wheat. Plant Soil 437:195–213
Farrag MMS, Toutou MMM, Sedik FS et al (2021) Towards the integrated agri-aquaculture in the desert using groundwater reservoirs for plants and nile tilapia farming “evaluating study in upper Egypt.” Egypt J Aquat Biol Fish 25:215–235. https://doi.org/10.21608/ejabf.2021.161839
Francois LE, Donovan T, Maas EV (1984) Salinity effects on seed yield, growth, and germination of grain sorghum 1. Agron J 76:741–744. https://doi.org/10.2134/agronj1984.00021962007600050008x
Franz S, Ehlert B, Liese A et al (2011) Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol Plant 4:83–96. https://doi.org/10.1093/mp/ssq064
Gabr MES (2022) Management of irrigation requirements using FAO-CROPWAT 8.0 model: a case study of Egypt. Model Earth Syst Environ 8:3127–3142. https://doi.org/10.1007/s40808-021-01268-4
Gengmao Z, Mehta SK, Zhaopu L (2010) Use of saline aquaculture wastewater to irrigate salt-tolerant Jerusalem artichoke and sunflower in semiarid coastal zones of China. Agric Water Manag 97:1987–1993. https://doi.org/10.1016/j.agwat.2009.04.013
Getachew G, Makkar HPS, Becker K (1999) Stoichiometric relationship between short chain fatty acids and in vitro gas production in presence and polythyleneglycol for tannin containing browses. In: EAAP, Satellite Symposium. pp 18–19
Gois GC, da Silva Matias AG, de Araújo GGL et al (2022) Nutritional and fermentative profile of forage sorghum irrigated with saline water. Biol Rhythm Res 53:246–257. https://doi.org/10.1080/09291016.2019.1629088
Guimarães MJM, Simões WL, De OAR et al (2019) Biometrics and grain yield of sorghum varieties irrigated with salt water Biometria e produção de variedades de sorgo granífero irrigado com águas salinas. Rev Bras Eng Agrícola Ambient 23:285–290
Guimarães MJM, Simões WL, Salviano AM et al (2022) Management for grain sorghum cultivation under saline water irrigation 1. Manejo da irrigação para o cultivo de sorgo granífero com água salina. Bras Eng Agrícola Ambient 26:755–762
Ha NTK, Em NT, Ngoc NM et al (2021) Effects of salinity on growth performance, survival rate, digestive enzyme activities and physiological parameters of striped catfish (Pangasianodon hypophthalmus) at larval stage. Can Tho Univ J Sci 13:1–9. https://doi.org/10.22144/ctu.jen.2021.011
Hadebe ST, Modi AT, Mabhaudhi T (2017) Drought tolerance and water use of cereal crops: a focus on sorghum as a food security crop in sub-Saharan Africa. J Agron Crop Sci 203:177–191. https://doi.org/10.1111/jac.12191
Hakim MA, Juraimi AS, Hanafi MM et al (2014) Biochemical and anatomical changes and yield reduction in rice (Oryza sativa L.) under varied salinity regimes. Biomed Res Int 2014:1–11
Hasibuan S, Syafriadiman S, Aryani N et al (2021) The age and quality of pond bottom soil affect water quality and production of Pangasius hypophthalmus in the tropical environment. Aquac Fish 8:296–304. https://doi.org/10.1016/j.aaf.2021.11.006
Hassan MU, Aamer M, Chattha MU et al (2020) The critical role of zinc in plants facing the drought stress. Agriculture 10:0396. https://doi.org/10.3390/agriculture10090396
Hedayati-Firoozabadi A, Kazemeini SA, Pirasteh-Anosheh H et al (2020) Forage yield and quality as affected by salt stress in different ratios of Sorghum bicolor-Bassia indica intercropping. J Plant Nutr 43:2579–2589. https://doi.org/10.1080/01904167.2020.1783301
Hieu DQ, Hang BTB, Huong DTT et al (2021) Salinity affects growth performance, physiology, immune responses and temperature resistance in striped catfish (Pangasianodon hypophthalmus) during its early life stages. Fish Physiol Biochem 47:1995–2013. https://doi.org/10.1007/s10695-021-01021-9
Holman JD, Obour AK, Mengel DB et al (2019) Nitrogen application effects on forage sorghum production and nitrate concentration. J Plant Nutr 42:2794–2804. https://doi.org/10.1080/01904167.2019.1659321
Hossain F, Islam SMM, Ashaf-Ud-Doulah M et al (2021) Influences of salinity on embryonic and larval development of striped catfish Pangasianodon hypophthalmus. Front Mar Sci 8. https://doi.org/10.3389/fmars.2021.781951
Huang J, Zhang HL, Tong WJ, Chen F (2012) The impact of local crops consumption on the water resources in Beijing. J Clean Prod 21:45–50. https://doi.org/10.1016/j.jclepro.2011.09.014
Hufnagel B, de Sousa SM, Assis L et al (2014) Duplicate and conquer: multiple homologs of PHOSPHORUS-STARVATION TOLERANCE1 enhance phosphorus acquisition and sorghum performance on low-phosphorus soils. Plant Physiol 166:659–677. https://doi.org/10.1104/pp.114.243949
Islam MA, Uddin MH, Uddin MJ, Shahjahan M (2019) Temperature changes influenced the growth performance and physiological functions of Thai pangas Pangasianodon hypophthalmus. Aquac Rep 13:100179. https://doi.org/10.1016/j.aqrep.2019.100179
Ismail Z, Go YI (2021) Fog-to-water for water scarcity in climate-change hazards hotspots: pilot study in Southeast Asia. Glob Challenges 5:2000036. https://doi.org/10.1002/gch2.202000036
Jahan A, Nipa TT, Islam SM et al (2019) Striped catfish (Pangasianodon hypophthalmus) could be suitable for coastal aquaculture. J Appl Ichthyol 35:994–1003. https://doi.org/10.1111/jai.13918
Kaplan M, Kamalak A, Kasra AA, Guven I (2014) Effect of maturity stages on potential nutritive value, methane production and condensed tannin content of Sanguisorba minor hay. Kafkas Univ Vet Fak Derg 20:445–449. https://doi.org/10.9775/kvfd.2013.10383
Kimera F, Mugwanya M, Dawood MAO, Sewilam H (2023) Growth response of kale (Brassica oleracea) and Nile tilapia (Oreochromis niloticus) under saline aqua‑sandponics‑vegeculture system. Sci Rep:1–12. https://doi.org/10.1038/s41598-023-29509-9
Kolozsvári I, Kun A, Jancsó M et al (2022) Agronomic performance of grain sorghum (Sorghum bicolor (L.) Moench) cultivars under intensive fish farm effluent irrigation. Agronomy 12:1185
Kumar A, Harikrishna V, Reddy AK et al (2017) Salinity tolerance of Pangasianodon hypophthalmus in inland saline water: effect on growth, survival and haematological parameters. Ecol Environ Conserv 23:567–574
Kumar A, Singh S, Gaurav AK, Srivastava S (2020) Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Front Microbiol 11:1–15. https://doi.org/10.3389/fmicb.2020.01216
Kumar A, Singh S, Mukherjee A et al (2021) Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol Res 242:126616. https://doi.org/10.1016/j.micres.2020.126616
Lamidi AA, Konyeha QG (2020) Agronomic indices and nutritional values of plant parts of three varieties of sorghum (Sorghum bicolor (L.) Moench) fodder at late stages of growth. Niger J Anim Prod 47:257–268
Liu C, Liu Y, Lu Y et al (2019) Use of a leaf chlorophyll content index to improve the prediction of above-ground biomass and productivity. PeerJ 6:e6240. https://doi.org/10.7717/peerj.6240
M.Moursy M, Rady M, Ali AMA et al (2019) Potentials of the use of brackish ground water in integrated aqua-agriculture systems. J Egypt Acad Soc Environ Dev D Environ Stud 20:117–130. https://doi.org/10.21608/jades.2019.68501
Mansour MMF, Emam MM, Salama KHA, Morsy AA (2021) Sorghum under saline conditions: responses, tolerance mechanisms, and management strategies. Planta, 254:1–38
Mariscal-Lagarda MM, Páez-Osuna F, Esquer-Méndez JL et al (2012) Integrated culture of white shrimp (Litopenaeus vannamei) and tomato (Lycopersicon esculentum Mill) with low salinity groundwater: management and production. Aquaculture 366–367:76–84. https://doi.org/10.1016/j.aquaculture.2012.09.003
Meena LL, Verma AK, Bharti VS et al (2022) Effect of foliar application of potassium with aquaculture wastewater on the growth of okra (Abelmoschus esculentus) and Pangasianodon hypophthalmus in recirculating aquaponic system. Sci Hortic (Amsterdam) 302:111161. https://doi.org/10.1016/j.scienta.2022.111161
Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev 28:7–55
Meritha WW, Suprayudi MA, Ekasari J (2018) The growth performance and resistance to salinity stress of striped catfish Pangasius sp. juvenile in biofloc system with different feeding rates Kinerja pertumbuhan dan ketahanan benih ikan patin terhadap stres salinitas dalam sistem bioflok dengan ting. J Akuakultur Indones 17:113–119. https://doi.org/10.19027/jai.17.2.113-119
Milford R, Minson DJ (1966) Determinants of feeding value of pasture and supplementary feed. Proc Aust Soc Anim Prod 6:319–329
Mishra BK, Kumar P, Saraswat C, Chakraborty S, Gautam A (2021) Water security in a changing environment: Concept, challenges and solutions. Water 13(4):490
Moghazy NH, Kaluarachchi JJ (2020) Assessment of groundwater resources in Siwa Oasis, Western Desert, Egypt. Alexandria Eng J 59:149–163. https://doi.org/10.1016/j.aej.2019.12.018
Mugwanya M, Kimera F, Dawood M, Sewilam H (2022) Elucidating the effects of combined treatments of salicylic acid and l-proline on greenhouse-grown cucumber under saline drip irrigation. J Plant Growth Regul. https://doi.org/10.1007/s00344-022-10634-0
Mugwanya M, Kimera F, Madkour K et al (2023) Influence of salinity on the biometric traits of striped catfish (Pangasianodon hypophthalmus) and barley (Hordeum vulgare) cultivated under an integrated aquaculture-agriculture system. BMC Plant Biol 23:1–18. https://doi.org/10.1186/s12870-023-04422-5
Nageswari P, Verma AK, Gupta S et al (2022a) Optimization of stocking density and its impact on growth and physiological responses of Pangasianodon hypophthalmus (Sauvage, 1878) fingerlings reared in finger millet based biofloc system. Aquaculture 551:737909. https://doi.org/10.1016/j.aquaculture.2022.737909
Nageswari P, Verma AK, Gupta S et al (2022b) Effects of different stocking densities on haematological, non-specific immune, and antioxidant defence parameters of striped catfish (Pangasianodon hypophthalmus) fingerlings reared in finger millet-based biofloc system. Aquac Int 3. https://doi.org/10.1007/s10499-022-00958-9
Nawaz M, Umair M, Muhammad H et al (2022) Trehalose: a promising osmo-protectant against salinity stress — physiological and molecular mechanisms and future prospective. Mol Biol Rep 49:11255–11271. https://doi.org/10.1007/s11033-022-07681-x
Nguyen PTH, Do HTT, Mather PB, Hurwood DA (2014) Experimental assessment of the effects of sublethal salinities on growth performance and stress in cultured tra catfish (Pangasianodon hypophthalmus). Fish Physiol Biochem 40:1839–1848. https://doi.org/10.1007/s10695-014-9972-1
Nhut N, Hao NV, Bosma RH et al (2019) Options to reuse sludge from striped catfish (Pangasianodon hypophthalmus, Sauvage, 1878) ponds and recirculating systems. Aquac Eng 87:102020. https://doi.org/10.1016/j.aquaeng.2019.102020
Noreen S, Sultan M, Salim M et al (2021) Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol Biochem 158:244–254. https://doi.org/10.1016/j.plaphy.2020.11.007
NRC (1989) National Research Council Nutrient Requirements of Dairy Cattle (6th rev. ed.), Natl. Acad. Sci., Washington, DC
Okomoda VT, Koh ICC, Hassan A et al (2019) Water quality tolerance and gill morphohistology of pure and reciprocal crosses of Pangasianodon hypophthalmus and Clarias gariepinus. J King Saud Univ - Sci 31:713–723. https://doi.org/10.1016/j.jksus.2018.01.003
Omotade IF, Alatise MO, Ollanrewaju OO (2019) Growth and yield performance of hot pepper using aquaculture wastewater. Agric Eng Int CIGR J 21:18–25
Oskey M, Velasquez C, Peña OM et al (2023) Yield, nutritional composition, and digestibility of conventional and brown midrib (BMR) pearl millet as affected by planting and harvesting dates and interseeded cowpea. Animals 13:260
Qiao T, Zhang L, Yu Y et al (2022) Identification and expression analysis of xyloglucan endotransglucosylase / hydrolase (XTH) family in grapevine (Vitis vinifera L.). PeerJ 10:13546. https://doi.org/10.7717/peerj.13546
Rashid AA (2012) Treatment of municipal pollution through re-engineered drains: a case study, Edfina drain, West Nile Delta. In: In 11th ICID, international drainage workshop (IDW), Cairo, Egypt
Saluja M, Zhu F, Yu H et al (2021) Loss of COMT activity reduces lateral root formation and alters the response to water limitation in sorghum brown midrib (bmr) 12 mutant. New Phytol 229:2780–2794. https://doi.org/10.1111/nph.17051
Sánchez-Duarte JI, Kalscheur KF, García AD, Contreras-Govea FE (2019) Short communication: meta-analysis of dairy cows fed conventional sorghum or corn silages compared with brown midrib sorghum silage. J Dairy Sci 102:419–425. https://doi.org/10.3168/jds.2018-14552
Sewilam H, Kimera F, Nasr P (2022) Water energy food nexus model: an integrated aqua-agriculture system to produce tilapia and sweet basil using desalinated water. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-23240-0
Shahjahan M, Uddin MH, Bain V, Haque MM (2018) Increased water temperature altered hemato-biochemical parameters and structure of peripheral erythrocytes in striped catfish Pangasianodon hypophthalmus. Fish Physiol Biochem 44:1309–1318. https://doi.org/10.1007/s10695-018-0522-0
Singh AK, Lakra WS (2012) Culture of Pangasianodon hypophthalmus into India: impacts and present scenario. Pakistan J Biol Sci 15:19
Song X, Zhou G, Ma BL et al (2019) Nitrogen application improved photosynthetic productivity, chlorophyll fluorescence, yield and yield components of two oat genotypes under saline conditions. Agronomy 9:115. https://doi.org/10.3390/agronomy9030115
Sriagtula R, Sowmen S, Aini Q (2019) Growth and productivity of brown midrib sorghum mutant line Patir 3.7 (Sorghum bicolor L. Moench) treated with different levels of nitrogen fertilizer. Trop Anim Sci J 42:209–214
Terkula B, Torsabo D, Che C et al (2023) A study on the recovery and characterization of suspended solid from aquaculture wastewater through coagulation / flocculation using chitosan and its viability as organic fertilizer. J Agric Food Res 11:100532. https://doi.org/10.1016/j.jafr.2023.100532
Tiika RJ, Wei J, Cui G et al (2021) Transcriptome-wide characterization and functional analysis of xyloglucan endo-transglycosylase/hydrolase (XTH) gene family of Salicornia europaea L. under salinity and drought stress. BMC Plant Biol 21:1–15. https://doi.org/10.1186/s12870-021-03269-y
Umesh MR, Lepcha I, Begna S et al (2022) Nutritive value and silage fermentation characteristics of forage sorghum (Sorghum bicolor L.) genotypes and Lablab (Lablab purpureus L.) mixture. Am J Plant Sci 13:723–733. https://doi.org/10.4236/ajps.2022.136048
Vacheron J, Desbrosses G, Bouffaud ML et al (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:1–19. https://doi.org/10.3389/fpls.2013.00356
Wahyono T, Indiratama WM, Human S (2021) White Midrib (WMR) vs Brown Midrib (BMR) sorghum: perspective of nutrient value for ruminant forage. In IOP Conference Series: Earth and Environmental Science (Vol. 788, No. 1, p. 012164). IOP Publishing
Wahyono T, Indriatama WM, Sasongko WT et al (2023) Forage-yield and nutrient quality of new brown midrib (BMR) mutant lines of sorghum. Trop Anim Sci J 46:63–73
Wahyono T, Sugoro I, Jayanegara A et al (2019) Nutrient profile and in vitro degradability of new promising mutant lines sorghum as forage in Indonesia. Adv Anim Vet Sci 7:810–818
Wang N, Zhao Z, Zhang X et al (2023) Plant growth, salt removal capacity, and forage nutritive value of the annual euhalophyte Suaeda salsa irrigated with saline water. Front Plant Sci 13:1–13. https://doi.org/10.3389/fpls.2022.1040520
Wasaya A, Manzoor S, Yasir TA et al (2021) Evaluation of fourteen bread wheat (Triticum aestivum L.) genotypes by observing gas exchange parameters, relative water and chlorophyll content, and yield attributes under drought stress. Sustainability 13:4799
Xuan Y, Sheng Z, Bo H, Min Z (2016) Ecotoxicology and environmental safety identification of a group of XTHs genes responding to heavy metal mercury, salinity and drought stresses in Medicago truncatula. Ecotoxicol Environ Saf 132:153–163. https://doi.org/10.1016/j.ecoenv.2016.06.007
Yang J, Ching YC, Chuah CH (2019) Applications of lignocellulosic fibers and lignin in bioplastics: a review. Polymers (Basel) 11:1–26. https://doi.org/10.3390/polym11050751
Zhou J, Li P, Wang J (2022) Effects of light intensity and temperature on the photosynthesis characteristics and yield of lettuce. Horticulturae 8:178
Zou Y, Zhang Y, Testerink C (2021) Root dynamic growth strategies in response to salinity. Plant Cell Environ 45:695–704. https://doi.org/10.1111/pce.14205
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by The American University in Cairo.
Author information
Authors and Affiliations
Contributions
H.S. and F.K. participated in conceptualization. F.K., M.M., K.M., and M.D contributed to research design and data collection. M.M contributed to data analysis and software. F.K., H.S., M.M., K.M., and M.D. contributed to paper writing, reviewing, and editing. H.S. supervised all the work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study followed the guidelines and approval of Committee of Animal Welfare and Research Ethics, Faculty of Agriculture, Kafrelsheikh University, Egypt.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Plant material
All plant materials and related procedures in this study were done in accordance with the guidelines of the Institutional Review Board of the American University in Cairo and the Ministry of Agriculture and Land Reclamation in Egypt.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kimera, F., Mugwanya, M., Madkour, K. et al. Maximization of brackish water productivity for the sustainable production of striped catfish (Pangasianodon hypophthalmus) and grain sorghum (Sorghum bicolor (L.) Moench) cultivated under an integrated aquaculture–agriculture system. Environ Sci Pollut Res 31, 31878–31895 (2024). https://doi.org/10.1007/s11356-024-33216-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33216-x