Abstract
Indoxacarb is one of the most extensively used oxadiazine insecticides worldwide, but it may exert detrimental effects on ecosystems, population dynamics, and health. Due to the lack of knowledge on the ecotoxicity of indoxacarb, it is still challenging to assess whether this insecticide poses an ecotoxicological impact on terrestrial environments. Therefore, our study aims to provide novel data on the toxic effects of 28-day dietary exposure to commercial grade indoxacarb at two environmentally relevant concentrations, 0.02 µg/mL and tenfold (0.2 µg/mL) on the model species, Theba pisana. Their effects were studied using a multiple biomarker approach by evaluating physiological, biochemical, and histopathological responses. After 28 days of treatment, indoxacarb at both concentrations significantly reduced the food intake and growth of the treated snails. Also, it caused decreases in lipid peroxidation (LPO) levels after 7 and 14 days of exposure, whereas an opposite effect occurred after 21 and 28 days. All treated snails were found to exhibit a lower content of glutathione (GSH) after all times of exposure. Moreover, catalase (CAT), glutathione-S-transferase (GST), and glutathione peroxidase (GPx) activities, as well as protein content (PC), were elevated in the treated snails after all time intervals. Post exposure to both realistic indoxacarb concentrations, changes in acetylcholinesterase (AChE) activity between a decrease and an increase were observed. Furthermore, indoxacarb caused histo-architectural changes in the hepatopancreas of T. pisana. Our results demonstrate that, at environmentally relevant concentrations, indoxacarb poses negative consequences for T. pisana, indicating its ecotoxicological impacts.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pesticide pollution associated with anthropogenic activities in the agricultural sector has increased worldwide. Pesticides are a class of hazardous chemicals deliberately applied to the environment to control various pests. Despite the fact that pesticides are vital to modern agriculture to boost agricultural output, their improper use has resulted in a significant environmental risk (Pathak et al. 2022). Consequently, some of these compounds can reach and accumulate in soil ecosystems and then become accessible for assimilation by terrestrial creatures (Sánchez-Bayo 2011). Given the ongoing environmental hazards from pesticide use, it is of utmost importance to assess whether this chemical poses an ecological risk to terrestrial environments. Among these compounds is the insecticide indoxacarb, which may put non-target terrestrial organisms at risk.
Indoxacarb, a promising new generation oxadiazine insecticide, is worldwide used to control sucking and chewing insects (Wing et al. 2000). It exhibits a novel mechanism of action by blocking the sodium channel, causing nervous system depression, paralysis, and death (Lapied et al. 2001). It has a moderate to strong tendency to partition from water to soil and consequently be relatively immobile in soil (U.S. EPA 2000). It is classified as having a moderate risk of bioaccumulation (Lin et al. 2023). The U.S. EPA (2000) reported that indoxacarb degrades well in soil under aerobic conditions (half-life 3 to 693 days). Its environmental profile indicates that considerable amounts of this compound can be found in terrestrial and aquatic environments. It was found that the initial residue of indoxacarb in soil was 0.27 mg/kg (Sdeek and Taha 2018) and in processed persimmons was 0.22 mg/kg (Hulbert et al. 2011). Despite indoxacarb has been shown to be safe for mammals (U.S. EPA 2000), the European Food Safety Authority has recently published a review and update addressing the risks associated with this pesticide to small mammals and bees (EFSA et al. 2018, EFSA 2019). In the soil, indoxacarb residues have exhibited low to moderate risk to arthropods and earthworms (Sakthiselvi et al. 2020). Moreover, it is classified as having a moderate to high risk to freshwater, estuarine/marine fish (Ghelichpour et al. 2019; Ren et al. 2021), aquatic insects (Monteiro et al. 2019), and soil algae (Patra et al. 2022).
Testing a commercial pesticide product, which is actually applied in the environment, is necessary to ascertain the true impact on non-target organisms and the environment because the formulated product’s toxicity is frequently greater than that of the active ingredient alone (Cossia et al. 2020). Excipients and an active ingredient are the two main components of pesticide formulations. Although they are considered inert chemicals due to their lack of pesticide activity, excipients found in commercial formulations can make pesticides more hazardous due to their toxic properties or by promoting the active ingredient’s bioavailability (Rozman et al. 2010). Furthermore, excipients’ impact can be significant to a commercial pesticide’s overall toxicity; so, it is necessary to consider their effects (Mesnage and Antoniou 2018). It is therefore more realistic to evaluate the toxicity of commercial formulations when examining the impact of pesticides on non-target organisms (Kovačević et al. 2023).
Because chemical monitoring is becoming less informative about ecological effects in ecotoxicological research, the use of biological models (bioindicators) helps directly determine the risk that these residues will actually be present in the terrestrial environment (Van Gestel 2012). Some land snail species are commonly used as bioindicators of soil quality. Based on Gomot de Vaufleury (2000) and Baroudi et al. (2020), land snails have the following characteristics: they are omnipresent and resident organisms, participate in the functioning of the ecosystem and food web, have a well-known ecophysiology, and exhibit remarkable tolerance to environmental pollution. They can also integrate pollution levels and subsequent ecotoxicological effects to gain information on environmental quality. Indeed, as they incorporate the three main routes of exposure, digestive, cutaneous, and pulmonary, land snails are useful tools for assessing risks worldwide (Gomot de Vaufleury and Pihan 2000). The land snail, Theba pisana, has the aforementioned characteristics that make it a good model for use as a bioindicator organism (Radwan et al. 2010; Louzon et al. 2023).
Biological markers, or biomarkers, are a sensitive biological technique for assessing pollution-induced stress (Marigomez et al. 2013). They can be evaluated at several biological organization levels (molecules, cells, individuals, and populations), giving them an integrative character of the entire chain of events that follow exposure to pollution (Vasseur and Cossu-Leguille 2003). Pesticides can alter the physiological homeostasis in a variety of taxa, including land snails, by inducing oxidative stress via reactive oxygen species (ROS) generation (Radwan et al. 2020; Qiao et al. 2022). Excessive production of ROS in tissues damages the structures of proteins, lipids, and DNA (Cattaneo et al. 2012). The metabolic process by which ROS cause oxidative damage to lipids is known as lipid peroxidation (LPO). This might have a substantial impact on the structure and function of cell membrane and, ultimately, cellular health (Vasilaki and McMillan 2017). To prevent oxidative damage, snails respond to stress by stimulating various antioxidant defense systems such as reduced glutathione (GSH), catalase (CAT), glutathione peroxidase (GPx), and glutathione-S-transferase (GST), which help maintain redox balance (Regoli et al. 2006; El-Gendy et al. 2019a). In addition, acetylcholinesterase (AChE) is an important enzyme involved in nerve signal transmission, and therefore, its alteration constitutes the neurotoxicity of contaminants (Fu et al. 2018). Moreover, histopathology, through the study of histo-architectural alterations in animal tissues demonstrates how pollutant-induced stress affects different tissues and organs (Hamed et al. 2007; Abo-Bakr 2011). In this context, physiological, biochemical, and histopathological markers are useful diagnostic tools and a cost-effective strategy for early-warning detection of environmental pollutants (Sogorb et al. 2014).
To the best of our knowledge, studies on the ecotoxicological consequences of indoxacarb on land snails have not yet been conducted. Therefore, this study aimed to investigate, for the first time, whether indoxacarb-based formulation at two environmentally relevant concentrations, 0.02 µg/mL and tenfold (0.2 µg/mL), poses negative effects on the model species, T. pisana. For this study, we selected a multiple biomarker approach by evaluating physiological, biochemical, and histopathological responses. Physiological manifestations were measured by feeding behavior and growth, and biochemical defects were assessed by parameters of oxidative stress: LPO, GSH, CAT, GPx, GST, and a neurotoxic biomarker, AChE, and protein content (PC) after 7, 14, 21, and 28 days of exposure. Histo-architectural alterations in the hepatopancreas of T. pisana were also estimated at the end of the experiment.
Materials and methods
Chemicals
The commercial indoxacarb (Truevaunt® 15% SC) used in this study was provided by EgyptChem International for Agrochemicals, Egypt. The chemical structure of indoxacarb is shown in Fig. 1. Thiobarbituric acid (TBA), trichloroacetic acid (TCA), metaphosphoric acid, 5,′5-dithio-bis-(2-nitrobenzoic acid) (DTNB), reduced glutathione (GSH), hydrogen peroxide (H2O2), cumene hydroperoxide, 1-chloro-2,4 dinitrobenzene (CDNB), acetylthiocholine iodide (ATChI), Folin-Ciocalteus phenol, and bovine serum albumin (BSA) came from Sigma-Aldrich Chemical Co., Germany.
Tested animals and adaptation
Specimens of 17.3 ± 0.07 mm in shell diameter and 0.94 ± 0.01-g adult terrestrial snail Theba pisana were collected in autumn from un-contaminated park in Alexandria , Egypt. In the acclimatization, the gathered snails were kept in ventilated cages (50 × 45 × 45 cm, 200 per cage) for at least 2 weeks prior to the trials in a laboratory setting (27 ± 2 °C, 64 ± 1% RH, and 14:10 L:D photoperiod). Animals were fed leaves of lettuce (Lactuca sativa) and starved for 48 h ahead of the experiment. The protocol of the study was approved by Alexandria University’s Animal Ethics Committee (AEC), and experiments were carried out in compliance with the care and handling instructions for animals.
Diet preparation
To evaluate the toxic effect of indoxacarb on T. pisana snails, an artificial food using dry ingredients that included 5-g rabbit meal, 3 g sucrose, and 2 g agar was made based on El-Gendy et al. (2011). In brief, indoxacarb at the requisite concentrations was added to 100 mL of agar medium to combine the dry ingredients. The obtained medium was equally distributed over four Petri dishes. Petri dishes were stored in the refrigerator after cooling. During the trials, a stainless-steel cork borer with a 3-cm diameter was used to make diet disks that were provided to the snails. The identical process was used to prepare the control diet, with the exception of distilled water rather than indoxacarb solution.
Experimental design
To reflect the environmental reality and based on the ecotoxicological profile of indoxacarb (EFSA et al. 2018; Sdeek and Taha 2018), we used two environmentally relevant indoxacarb concentrations: 0.02 and 0.2 µg/mL. Individuals of T. pisana were subjected to both concentrations in their diets as described before for 7, 14, 21, and 28 days. In this test, 225 snails (75 individuals/treatment, 3 reps) were separated into 3 categories; the first category acted as the control group and was fed a non-toxic diet. The second category of snails received food treated with 0.02 µg/mL indoxacarb while the third category received food treated with 0.2 µg/mL indoxacarb.
Exposure impact on physiological parameters
Feeding and growth responses
Snails of three sets (60 individuals/set, 3 reps) were kept in 12-cm-diameter plastic boxes, where they were given fresh agar disks up to 28 days. For the course of the trial, the boxes were examined every day, water was sprayed on the snails daily to keep them wet, and the diet was replaced every 48 h. Before giving fresh agar disks to the snails, the weight of the disks was measured, and any leftover diet was gathered and dried at 60 °C until it reached a constant weight. It was therefore possible to determine how much dry matter the dried disks actually contained by weighing them, which allowed for the calculation of the snails’ dry matter consumption. The mean weight (g) of dry food ingested per animal per time interval was used to calculate the feeding rate. According to Gomot de Vaufleury (2000), the snail mass was determined by weighing each animal at the beginning of the experiment and again after 7, 14, 21, and 28 days in order to calculate the change in animal mass (g).
Exposure impact on biochemical parameters
Eighteen snails were chosen at random from each replicate after each time interval and anesthetized using 5% ethanol (d’Ovidio et al. 2019). The snail tissues (hepatopancreas and head-foot) of the selected snails were quickly dissected, cleaned with 0.9% cold saline, weighed, and homogenized with 5 volumes of cold saline for 60s by a Polytron homogenizer (Tekmar tissumizer, Ohio, USA). Using a cooling centrifuge (Janetzki K23 centrifuge, Engelsdorf-Leipzig, Germany), the homogenate was centrifuged at 5000 × g for 20 min at 4 °C. LPO and GSH levels were measured in the homogenate of the hepatopancreas. CAT, GPx, and GST activities, and PC were estimated in the hepatopancreas supernatant, while AChE activity was assessed in the head-foot region.
LPO level
Malondialdehyde (MDA) production was quantified at 535 nm using the thiobarbituric acid (TBA) technique established by Placer et al. (1966). Briefly, 0.33 mL of hepatopancreas homogenate was added to 3 mL of freshly prepared TBA reagent consisting of 1:3 volumes of 0.8% TBA and 20% trichloroacetic acid (TCA), respectively. After boiling for 20 min, the mixture was cooled and centrifuged at 3000 g for 20 min. The MDA level was measured spectrophotometrically using a T-80 + UV/VIS spectrometer PG Instrument Ltd., United Kingdom at 532 nm. The level of LPO is denoted as nmol MDA/g wet tissue.
GSH content
Using the method outlined by Owens and Belcher (1965), the content of GSH was measured at 412 nm using 5,′5-dithio-bis-(2-nitrobenzoic acid) (DTNB). The assay mixture contained the homogenate (0.1 mL), 0.5 M phosphate buffer, pH 8.0 (1.5 mL), 3% metaphosphoric acid (0.4 mL), and 0.01 M DTNB (30 µL). After calibration using the standard GSH curve, its content was presented as mg/g wet tissue.
CAT activity
The activity of CAT was estimated at 240 nm as illustrated by Beers and Sizer (1952). An aliquot from the hepatopancreas supernatant was mixed with 2 mL of phosphate buffer (66.7 mM), pH 7. The assay was started by adding 1 mL of 12.5 mM hydrogen peroxide (substrate), which was freshly prepared. The time (Δt) needed for a 0.05 decrease in absorbance to be observed is used for the calculation. The activity is represented in unit/g wet tissue.
GPx activity
GPx activity was assessed according to Chiu et al. (1976). One-hundred microliters of supernatant was mixed with a mixture containing 100 µL of GSH (1 mM), 100 µL of cumene hydroperoxide (0.05%), and 50 µL of DTNB (0.01 M), and 2.65 mL of Tris–HCl buffer, pH 8.9 (0.4 M). After 1 min, the developed yellow color was measured at 412 nm against a blank (without DTNB). The enzyme activity was displayed as nmol/mg protein.
GST activity
The activity of GST was determined as previously described by Vessey and Boyer (1984). The reaction mixture consisted of 10 μL supernatant, 20 µL of 37.5 mM 1-chloro-2,4 dinitrobenzene (CDNB) solution as a substrate, and 0.2 mL of 4 mM GSH solution, and then the volume was completed to 3 mL with 0.1 M phosphate buffer, pH 7.0. The mixture was incubated for 20 min at room temperature, and the absorbance was then measured at 340 nm. The enzyme activity was expressed as μmol/min/mg protein.
AChE activity
The activity of AChE was measured based on the Ellman et al. (1961) procedure. The 2.8 mL of 0.1 M phosphate buffer (pH 8), 0.1 mL of head-foot supernatant, and 0.1 mL of DTNB solution made up the reaction mixture. To this mixture, 0.02 mL of freshly prepared acetylthiocholine iodide (ATChI) solution was added. After 10 min, the absorbance was measured at 412 nm. The AChE activity was presented as μmol/min/mg protein.
PC
Using bovine serum albumin (BSA) as a standard, protein content was measured in accordance with Lowry et al. (1951). Ten microliters of supernatant was added to 3 mL of medium freshly prepared by mixing 50 mL of reagent A (2 g Na2CO3 in 100 mL of 0.1 N NaOH) with 1 mL of reagent B (0.5 g CuSO4.5H2O in 100 mL of a solution containing 1 g sodium potassium tartrate). The mixture was mixed well and incubated for 10 min at room temperature. 0.3 mL of Folin-Ciocalteus phenol solution was added and mixed well. After 30 min, the developed blue color was estimated by recording the intensity at 750 nm.
Exposure impact on histopathological alterations
At the end of the trial (28 days), the hepatopancreas of three animals from either the control group or those exposed to the tested concentrations of indoxacarb were dissected. The hepatopancreas that had been dissected were preserved for a minimum of 24 h in 10% formalin. The fixative solution was washed overnight under running water. After passing via a series of alcohol solutions, the tissue was dried, embedded with wax, cut into 4-μm pieces, mounted on a slide, and dyed with hematoxylin and eosin (Banchroft et al. 1996). The cytological examinations were conducted under 400 × magnification using a Leica DM500 optical microscope, Heerbrugg, Switzerland and photomicrographs were taken in a bright field.
Data analysis
Data were displayed in all measurements as mean ± standard error (SE). Shapiro–Wilk and Levene’s tests were used to check the results of the parameter tests for normality and homogeneity of variance, respectively. The ANOVA analysis of the data was followed by the separation of the means using the Student–Newman–Keuls test at a probability level (p ≤ 0.05). Software Costat V. 2.6 (Costat program 2002) was used for the statistical analysis.
Results
In the current study, the feeding behavior and growth response of indoxacarb-treated snails were examined as physiological biomarkers to detect any changes that occurred in the animals’ physiology.
Exposure impact on physiological parameters
Feeding behavior
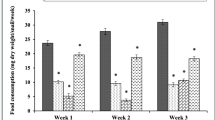
The effect of dietary indoxacarb at 0.02 and 0.2 µg/mL on food consumption was noticeable after 7 days of exposure (Fig. 2A). Food intake by snails was significantly decreased after 21 and 28 days of indoxacarb exposure. After 21 days, the control group had ingested 1.14 g dry food/snail, whereas this amount significantly dropped to 0.94 and 0.84 g in the case of snails treated with 0.02 and 0.2 µg/mL of indoxacarb, respectively. Also, the food consumption after 28 days significantly decreased from 1.17-g dry food (control group) to 0.80-g dry food for snails treated with low concentration, whereas it decreased to 0.68-g dry food in snails treated with high concentration of indoxacarb. Moreover, it was observed that the effect of indoxacarb at high concentration on food consumption was more pronounced than at low concentration throughout the experimental exposure periods.
Growth response
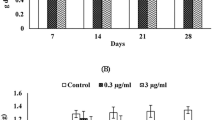
The mass (represented as mean body weight gain) of control snails was gradually increased throughout the experimental periods. For animals fed on an indoxacarb-contaminated diet, their average mass increased from the beginning of the experiment up to 14 days and then significantly decreased after 21 and 28 days of exposure compared with the control. The mass of snails was 0.99 and 0.92 g when they fed on a 0.02 µg/mL indoxacarb-intoxicated diet and 0.79 and 0.71g on a 0.2 µg/mL indoxacarb-contaminated diet, compared to 1.32 and 1.34 g for the control, after 21 and 28 days, respectively. Overall, the mass of snails subjected to high indoxacarb concentration was less than that of snails exposed to low concentration during 28 days of exposure (Fig. 2B).
Exposure impact on biochemical parameters
LPO level
The obtained results revealed that the LPO level in snails exposed to indoxacarb at the two tested concentrations was non-significantly decrease after 7 and 14 days of exposure. Indoxacarb at 0.02 µg/mL caused an enhancement in LPO level with non-significant differences, however, at 0.2 µg/mL resulted in a significant induction in this respect after 21 days of exposure. Compared to the control sample (1.20 nmol MDA/g.w.t), indoxacarb at low concentration (1.92 nmol MDA/g.w.t) and indoxacarb at high concentration (2.27 nmol MDA/g.w.t) significantly elevated the LPO level after 28-day exposure (Table 1).
GSH content
Data in Table 2 exhibited that both tested concentrations of indoxacarb insignificantly depressed the GSH content of the animal after 7 and 14 days of treatment. Also, it was demonstrated that no significant decrease in GSH content of snails treated with 0.02 µg/mL indoxacarb was observed after 21 days, whereas snails treated with 0.2 µg/mL indoxacarb exhibited a significant decrease in this context. After 28 days, indoxacarb at both tested concentrations caused a significant decrease in GSH content where the mean value of GSH was 5.35 mg/g.w.t in snails exposed to a low concentration and 5.21 mg/g.w.t in snails exposed to a high concentration compared to the control value (5.90 mg/g.w.t).
CAT activity
Results clearly indicated that indoxacarb at both concentrations (0.02 and 0.2 µg/mL) significantly enhanced the CAT activity throughout the experimental time in comparison to the control. The activity of CAT in snails exposed to 0.02 µg/mL indoxacarb was elevated by 114.44, 116.23, 142.13, and 131.15% after 7, 14, 21, and 28 days, respectively. Also, the enzyme activity of snails exposed to 0.2 µg/mL indoxacarb was enhanced by 120.78, 122.82, 148.80, and 138.13%, respectively. During the experimental period, indoxacarb at a concentration of 0.2 µg/mL was found to has a greater effect on the CAT activity than at a concentration of 0.02 µg/mL (Table 3).
GPx activity
The treatment of snails with indoxacarb at 0.02 µg/mL gave a non-significant elevation in GPx activity while the enzyme activity of snails treated with 0.2 µg/mL was significantly increased after 7 days of exposure. Furthermore, indoxacarb at both concentrations caused a significant increase in the activity of GPx after 14 and 21 days of treatment. The mean figures of GPX activity in animals subjected to 0.02 and 0.2 µg/mL indoxacarb were 122.75 and 129.62 nmol/mg protein, respectively, in comparison to the control value (96.35 nmol/mg protein) post14 days. The corresponding values were 170.93 and 178.98 nmol/mg protein, respectively, in comparison to the control value (129.30 nmol/mg protein) post21 days. Both tested indoxacarb concentrations, however, caused a non-significant elevation in the GPx activity of exposed snails after 28 days of exposure (Table 4).
GST activity
As shown in Table 5, a non-significant elevation in the activity of GST in snails subjected to indoxacarb at 0.02 µg/mL; however, a significant induction in the enzyme activity of the snails exposed to 0.2 µg/mL after 7 and 14 days of exposure was observed. After 21 and 28 days, indoxacarb at two tested concentrations induced a significant augmentation in GST activity of snails. The data also showed that the enhancement of GST activity was time- and concentration-dependent.
AChE activity
The results showed a significant increase in AChE activity for both tested concentrations (0.02 and 0.2 µg/mL) after 7 and 14 days when compared to control snails. However, the opposite trend was observed after 21 and 28 days, where the AChE activity was significantly inhibited. AChE activities of snails exposed to a low concentration of indoxacarb were 205.53, 271.70, 73.71, and 65.96% of control after 7, 14, 21, and 28 days of exposure, respectively. The corresponding values of a high concentration were 209.48, 366.94, 67.42, and 60.04% of control. Generally, a high concentration of indoxacarb was found to be more affected on the AChE activity than a low one (Fig. 3).
PC
The results clearly indicated that both tested indoxacarb concentrations significantly increased the PC of treated animalsafter 7 and 14 days of exposure. Snails treated with 0.02 µg/mL indoxacarb exhibited a non-significant increase in PC, while the treatment with 0.2 µg/mL indoxacarb gave a significant decrease in this respect after 21 days of exposure. A non-significant elevation in PC was observed in exposed snails to indoxacarb at both tested concentrations after 28 days of exposure (Fig. 4).
Exposure impact on histopathological alterations
Figure 5A, B, and C show the photomicrographs of control and indoxacarb-treated snail hepatopancreas.
Control group
The digestive cells (DC), digestive tubules (DT), excretory cells (EC), lumen (L), calcium cells (C), nucleus (N), and muscle layer (ML) of the un-exposed snails had a normal manifestation, showing no histopathological alterations (Fig. 5A).
Experimental groups
In the case of snails subjected to 0.02 µg/mL indoxacarb, the obtained observations displayed various histo-architectural damages, as shown in digestive cells occupied by digestive granules, and the excretory cells were filled with huge vacuoles containing excretory granules of variable size and increased the layer of muscle fiber (Fig. 5B). On the other hand, the group of snails subjected to 0.2 μg/ml indoxacarb exhibited, disruption of digestive tubules, rupture of digestive cells, increase of vacuoles, and the presence of excretory cells with excretory granules of variable size and clumps (Fig. 5C).
Discussion
The effect of pesticides on environmental compartments has recently become a significant issue on a global scale. Continuous use of pesticides burdens the soil ecosystem, leads to a decline in its health, and may harm soil-dwelling invertebrates that serve as bio-indicators of soil quality. Consequently, more in-depth ecotoxicological data are required for a better grasp of their real risks, as the usage of pesticides is unlikely to decrease in the foreseeable future (Gunstone et al. 2021). Up to now, the eco-toxic effects of indoxacarb on T. pisana snails have not been addressed. To the best of our knowledge, this is the pioneer investigation of utmost importance to find out whether indoxacarb-based formulation poses an ecological consequence to the non-target gastropod, T. pisana. Therefore, some consequences were evaluated for 28-day exposure to indoxacarb at two environmentally relevant concentrations to elucidate snail physiological, biochemical, and histopathological responses.
In the current study, changes in feeding behavior and growth response of treated snails with indoxacarb were examined as physiological biomarkers. The feeding behavior constitutes one of the earliest reactions to environmental perturbations (McLoughlin et al. 2000), and subsequently, effects on an organisms’ ability to feed may be interpreted into effects on growth, and eventually on populations (Slijkerman et al. 2004). Land gastropods in the wild consume a variety of foods, including dead animal prey and litter, which lead to the accumulation of harmful chemicals in its bodies (Dallinger et al. 2001). Feeding behavior in land gastropods is controlled by the central nervous system, and substantial research is being done to understand how this is accomplished (Chase 2002).
Our findings show that dietary exposure to the two realistic indoxacarb concentrations up to 28 days has been found to reduce the food consumption of snails, indicating physiological stress. The reduced food intake of treated snails might be a result of the indoxacarb neurotoxic effect, which alters their feeding behavior. Substantial reductions in the food intake of snails fed on pollutant-contaminated diets have been reported by many researchers; Gomot de Vaufleury (2000) reported that the food consumption rate of Canterus aspersus, subjected to a pentachlorophenol-contaminated diet was sharply decreased. The feeding rate of C. aspersus was also significantly reduced after dimethoate exposure (Coeurdassier et al. 2001). Also, significant decreases in the food consumption rate of T. pisana were noticed after 2 weeks of exposure to 0.05 LC50 of thiamethoxam, abamectin, and acrylamide (El-Gendy et al. 2019b). Furthermore, following 14 days of exposure, T. pisana snails consumed significantly less food contaminated by boric acid (Radwan and Gad 2023).
Another potential indictor of physiological integrity is growth measurement (Conti 2008). This index is regarded as a crucial measure of how energetically healthy an organism is under the stress of contamination (Widdows et al. 1997). Various physiological processes can affect growth rate, including alterations in food ingestion, availability, assimilation, and nutrient uptake (Sanders et al. 2018).
In the present study, the growth reduction of the treated snails may be due to the tested compound interference with the feeding activities of these animals. It is possible that decreases in the rate of food consumption may be reflected in decreases of snail total mass.
Our results are consistent with those of Coeurdassier et al. (2001) who established that the growth characteristics of C. aspersus decreased after 4 weeks of dimethoate exposure. Moreover, T. pisana growth was diminished after dietary exposure to a contaminated diet with thiamethoxam, abamectin, and acrylamide for 14 days (El-Gendy et al. 2019b). Additionally, the growth of T. pisana snails was significantly reduced after being fed sub-lethal concentrations of boric acid for 14 days (Radwan and Gad 2023).
It is known that the hepatopancreas is the primary gastropod organ to be impacted by the body’s intake pathway for xenobiotics and is involved in accumulation, detoxification processes, and elimination of pollutants (Dallinger et al. 2001; Radwan et al. 2020). In our study, exposure of T. pisana snails to indoxacarb likely induced ROS. This is evidenced by varying stress markers in terms of LPO levels and antioxidant enzyme activities. Therefore, the biochemical and histo-architectural impacts of indoxacarb exposure are predominantly evaluated in the snail hepatopancreas.
LPO is considered a crucial indicator for assessing oxidative stress responses to different xenobiotics. It is a process by which free radicals attack the poly-unsaturated fatty acids of the cellular membrane leading to membrane dysfunction (Halliwell and Gutteridge 2007). The end product of LPO, malondialdehyde (MDA), is employed as a marker of oxidative stress and tissue damage in gastropods (Livingstone et al. 1990). In the current study, the elevation of LPO levels in intoxicated snails may be caused by an excess of ROS or a decrease in the activity of the antioxidant system, suggesting that there is some cellular oxidative injuries as a result of an imbalance in the antioxidative processes (Prased and Muralidhara 2012). On the other hand, the decrease in LPO levels indicated in our investigation can be due to the protective role of the antioxidant system in decreasing LPO levels resulting from ROS. The outcomes of our investigation coincide with preceding findings of Gamil et al. (2011) who revealed a decrease in LPO level after 24 h in second- and fourth-instar larvae of Spodoptera littoralis exposed to indoxacarb. In another study, an increase in LPO level was reported in male albino rats after 21-day exposure to indoxacarb (Hassan et al. 2021). Further, Monteiro et al. (2019) reported that exposure of the aquatic midge, Chironomus riparius, to 1, 2, 4, and 8 μg/L indoxacarb caused an increase in the level of LPO after 48 h of treatment.
GSH, a low molecular weight tripeptide, plays a crucial role in the detoxifying process. It functions either directly via forming conjugates with electrophilic intermediates or free radicals (Van der Oost et al. 2003) or indirectly via serving as a co-substrate for specific antioxidant enzymes like GST and GPx (Storey 1996). In the current study, the tested compound caused a reduction in the level of GSH in the hepatopancreas of T. pisana. This finding could be attributed to radical-induced oxidation and implies an imbalance between antioxidants and oxyradicals that eventually results in oxidative damage (Halliwell and Gutteridge 2007), the pathway of GSH production was negatively affected by xenobiotic stresses (Peña-Llopis et al. 2002), or the consumption of GSH is associated with an elevation of the enzyme activity of GST (Canesi et al. 1999). Accordingly, our findings completely agreed with the results of Sbartai et al. (2012) who noted that the GSH content decreased in the freshwater ciliated protozoa Paramecium sp. from 48 up to 96 h of exposure to indoxacarb. Moreover, a decrease in GSH level in the German cockroach, Blattella germanica, treated with LD50 and LD90 of indoxacarb after 72 h was observed (Maiza et al. 2013).
CAT is an intracellular antioxidant enzyme that catalyzes the conversion of hydrogen peroxide (H2O2) into O2 and H2O in order to avoid cellular oxidative damage (Akyilmaz and Dinckaya 2003). In the present study, it was found that CAT activity in T. pisana snails was markedly increased, indicating a compensatory elevation in CAT activity to cope with the enhancement of ROS production caused by a pesticide for protecting the cells from oxidative stress conditions (Torres et al. 2002). From the literature survey about activity of the CAT enzyme in organisms exposed to pollutants, contradictory findings have been found. An increase in CAT activity was reported by some authors, while a decrease in its activity was reported by others. Paramecium sp., exposed to different concentrations (10, 20, 40, and 80 μm) of indoxacarb, resulted in an increase in CAT activity from 1 to 96 h of treatment (Sbartai et al. 2012). Similarly, indoxacarb enhanced CAT activity in the exposed gammaridean arthropod, Gammarus kischineffensis after 48, 72, and 96 h (Demirci et al. 2018). Recently, Ren et al. (2021) found that CAT activity was increased in zebrafish after 28-day exposure to indoxacarb (0.01 mg/L). Indoxacarb exposure decreased the CAT activity of C. riparius at concentrations of 2 and 4 μg/L, but increased at the highest tested concentration (8 μg/L) after 48 h (Monteiro et al. 2019).
GPx is an important intracellular antioxidant enzyme for the detoxification of H2O2 to water to limit its harmful effects (Orbea et al. 2000). Also, GPx stimulates the oxidation of reduced GSH to oxidized glutathione disulfide (GSSG) to facilitate the transformation of peroxides to alcohol (Regoli and Giuliani 2014). In our study, the increased activity of GPx in T. pisana snail’s dietary exposed to indoxacarb may be due to the excess of free radical’s production that can stimulate the activity of GPx to scavenge peroxides. Our observations are in harmony with the data published by Monteiro et al. (2019) who showed that an induction in activity of GPx was noticed in C. riparius subjected to 1, 2, 4, and 8 μg/L of indoxacarb after 48 h. Likewise, indoxacarb exposure for 20 days caused an increase in the GPx activity of Ostrinia nubilalis (Franeta et al. 2018).
GST is a phase II multifunctional enzyme that scavenges ROS in order to preserve the cells against oxidative damage. Additionally, it has a detoxifying role, which can be binding with lipophilic hazardous substances to transform them into hydrophilic substances that are then excreted through metabolic processes (Atamaniuk et al. 2014). In the current research, it was found that the activity of GST in the intoxicated snails increased throughout the exposure periods. The generation of ROS after exposure to the tested compound and the stimulation of the antioxidant defense mechanism by the toxic substance may account for the induction of GST activity (Elia et al. 2007). Our results are corroborated by the work of Monteiro et al. (2019) who reported that indoxacarb increased GST activity in the exposed C. riparius after 48 h. An increased GST activity was also observed in experiments with second- and fourth-instar S. littoralis larvae exposed to LC50 of indoxacarb for 24 h (Gamil et al. 2011). Augmentation in GST was also reported after exposure of B. germanica to LD50 and LD90 of indoxacarb for 72 h (Maiza et al. 2013). Chronic exposure for 20 days to indoxacarb caused a significant enhancement in the GST of O. nubilalis (Franeta et al. 2018).
AChE is frequently employed as a reliable neurological endpoint for many environmental contaminants. It plays an essential role in the nervous system via hydrolyzing acetylcholine (ACh), which acts as a neurotransmitter (Lionetto et al. 2013). In our investigation, indoxacarb caused an augmentation in the AChE activity at some exposure times, suggesting a negative impact on the snail’s nervous system. The present finding suggests that the tested pesticide increased AChE activity in T. pisana snails due to oxidative stress, which was manifested as LPO. In a related study, it was noted that AChE stimulation in the rat brain was associated with a deterioration in the antioxidant status (Carageorgiou et al. 2005). However, it caused suppression of AChE activity in the other exposure intervals, indicating that the tested compound has a neurotoxic action on treated snails. The inhibitory mechanisms may suggest that AChE is unable to hydrolyze acetylcholine . This can lead to the accumulation of ACh, resulting in hyperstimulation, loss of muscular control, and ultimately death (Fulton and Key 2001). AChE activity was also discovered to be particularly sensitive to the effects of free radical (H2O2) in the 1.6–6.4-μM range, suggesting that a decline in its activity could be related to the impact of a high amount of H2O2 produced by the LPO pathway (Danylovych 1999). Our results are consistent with earlier research that showed that indoxacarb exposure for 48 h increased the AChE activity of C. riparius (Monteiro et al. 2019). In the study of Demirci et al. (2018), AChE activity was found to increase in G. kischineffensis subjected to 1/100 LC50 indoxacarb after 24-, 48-, 72-, and 96-h exposure. Furthermore, Abdel-Halim et al. (2006) showed that sub-lethal exposure to 0.5 LC50 and LC50 indoxacarb inhibited the activity of AChE in the soft tissues of the land snail, Monacha cartusiana, after 48 h. Inhibition of AChE activity was also observed in B. germanica exposed to LD50 and LD90 indoxacarb for 72-h exposure (Maiza et al. 2013).
Proteins are substantial organic compounds needed by living organisms for tissue construction. They also play a part in cell architecture and energy metabolism. Proteins regulate the interaction process between intracellular and extracellular media (Remia et al. 2008). In the present study, the elevation of PC observed in the intoxicated snails might be the result of a rise in protein synthesis in response to indoxacarb stress. Our results are in accordance with Gamil et al. (2011) who indicated that the PC of S. littoralis second-instar larvae treated with indoxacarb increased, whereas the PC of the fourth-instar larvae showed a decrease in comparison to the control.
Our physiological and biochemical data were supported by histopathological observations. Histopathology is a key component of the toxicological and risk assessment of xenobiotics. It is a sensitive, rapid, and valuable marker to demonstrate how xenobiotic-induced sub-lethal stress affects different tissues and organs through the study of histo-architectural alterations in animal tissues, which may also be used to detect environmental hazards from chemicals (Hamed et al. 2007; Radwan et al. 2008). All the histopathological observations in the current investigation indicated that treated snails displayed histo-architectural damage to the hepatopancreas tissues due to indoxacarb stress. Indoxacarb has not been examined for its histopathological impacts on land gastropods, although several injuries to the hepatopancreas of snails caused by several pesticides have been documented by some researchers: Hamed et al. (2007) showed histo-architectural alterations in the hepatopancreas of Eobania vermiculata subjected to methiocarb or methomyl using the baiting technique. Both pesticides resulted in significant cytoplasmic vacuolization and disruption with the lowering of microvilli, surface blab formation, an increase in the calcium spherule numbers in calcium cells, and an irregular increase in excretory cell numbers with a lot of excretory granules or residual bodies. After 96 h of exposure, Gaber et al. (2022) revealed that LC20 and LC40 of methomyl had caused notable degeneration and ruptured digestive cells, some ruptured excretory cells, and the replacement of the cytoplasm in other cells at LC20 concentration in M. cartusiana.
Conclusions
The scientific novelty of our research study lies in the fact that for the first time, we evaluated the reactions of the non-target T. pisana species to the commercial indoxacarb product by evaluating physiological, biochemical, and histopathological markers to assess the ecotoxicological impact. In this study, it can be concluded that dietary exposure of snails to environmentally relevant concentrations of indoxacarb-based product evokes an adverse impact on animal physiology, oxidative stress and neurotoxicity and caused histo-architectural changes in the hepatopancreas of snails. These tested parameters in snails can be considered useful biomarkers for the diagnosis of indoxacab contamination in terrestrial ecosystems. Since T. pisana responds to indoxacarb exposure via biological alterations, it could provide a reliable bioindicator species to monitor the ecological consequences of the compound. Nevertheless, other new and reliable biomarkers, such as omics, need to be identified to provide more risk assessment information on indoxacarb and/or its metabolites in diverse ecosystems. Finally, to minimize the adverse effects arising from indoxacarb exposure, management programs through regulatory bodies should be implemented to prevent ecosystem disruption and the consequences for non-target organisms.
Data availability
All data analyzed during this study are included in this article.
The raw data that support the findings of this study are available on request from the corresponding author.
References
Abdel-Halim KY, Abou-El Khear RK, Hussein AA (2006) Molluscicidal efficacy and toxicity of some pesticides under laboratory and field conditions. Arab Univ J Agric Sci, Ain Shams Univ, Cairo 14:861–870
Abo-Bakr Y (2011) Histopathological changes induced by metaldehyde in Eobania vermiculata (Müller 1774). Alex Sci Exch J 32:300–310
Akyilmaz E, Dinckaya E (2003) Development of a catalase based biosensor for alcohol determination in beer samples. Talanta 61:113–118
Atamaniuk TM, Kubra OI, Husak VV, Storey KB, Lushchak VI (2014) The Mancozeb-containing carbamate fungicide tattoo induces mild oxidative stress in goldfish brain, liver, and kidney. Environ Toxicol 29:1227–1235
Banchroft JD, Stevens A, Turner DR (1996) Theory and practice of histological techniques, 4th edn. Churchil Livingstone, New York, London, San Francisco, Tokyo
Baroudi F, Al Alam J, Fajloun Z, Millet M (2020) Snail as sentinel organism for monitoring the environmental pollution, a review. Ecol Indic 113:106240
Beers RF Jr, Sizer IW (1952) Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Canesi L, Viarengo A, Leonzio C, Filippelli M, Gallo G (1999) Heavy metals and glutathione metabolism in mussel tissues. Aquat Toxicol 46:67–76
Carageorgiou H, Tzotzes V, Sideris A, Zarros A, Tsakiris S (2005) Cadmium effects on brain acetylcholinesterase activity and antioxidant status of adult rats: modulation by zinc, calcium and L-cysteine co-administration. Basic Clin Pharmacol Toxicol 97:320–324
Cattaneo R, Moraes BS, Loro VL, Pretto A, Menezes C, Sartori GMS, Clasen B, de Avila LA, Marchesan E, Zanella R (2012) Tissue biochemical alterations of Cyprinus carpio exposed to commercial herbicide containing Clomazone under rice-field conditions. Arch Environ Contam Toxicol 62:97–106
Chase R (2002) Behavior and its neural control in gastropod molluscs. Oxford University Press, New York, NY
Chiu DTY, Stults FH, Tappel AL (1976) Purification and properties of rat lung soluble glutathione peroxidase. Biochim Biophys Acta 445:558–566
Coeurdassier M, Saint-Denis M, Gomot de Vaufleury A, Ribera D, Badot PM (2001) The garden snail (Helix aspersa) as a bioindicator of organophosphorus exposure: effects of dimethoate on survival, growth, and acetylcholinesterase activity. Environ Toxicol Chem 20:1951-1957
Conti ME (2008) Biological monitoring: theory and applications: bioindicators and biomarkers. Technology and Engineering, pp 228
Cossia PF, Herbert LT, Yusseppone MS, Perezd AF, Kristoffa G (2020) Toxicity evaluation of the active ingredient acetamiprid and a commercial formulation (Assail® 70) on the non-target gastropod Biomphalaria straminea (Mollusca: Planorbidae). Ecotoxicol Environ Saf 192:110248
Costat program (2002)Microcomputer program analysis. CoHort software, Version 2.6, Monterey, CA
Dallinger R, Be B, Triebskorn R, Köhler H (2001) Soil biology and ecotoxicology. In: Barker GM (ed) The Biology of Terrestrial Molluscs. CABI Publishing, New York, pp 489–525
Danylovych IV (1999) Hydrogen peroxide inhibits acetylcholinesterase of myometrium sarcolemma. Ukr Biokhim Zh 8:32–38
Demirci Ö, Güven K, Asma D, Öğüt S, Uğurlu P (2018) Effects of endosulfan, thiamethoxam, and indoxacarb in combination with atrazine on multi-biomarkers in Gammarus kischineffensis. Ecotoxicol Environ Saf 147:749–758
d’Ovidio D, Monticelli P, Santoro M, Adami C (2019) Immersion anaesthesia with ethanol in African giant land snails (Acathina fulica). Heliyon 5(4):e01546
EFSA (2019) Updated peer review concerning the risk to mammals and bees for the active substance indoxacarb. European Food Safety Authority 17:5866. https://doi.org/10.2903/j.efsa.2019.5866
EFSA, Arena M, Auteri D, Barmaz S, Bellisai G, Brancato A, Brocca D, Bura L, Byers H, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Magrans JO, Medina P, Miron I, Molnar T, Nougadere A, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Verani A, Villamar-Bouza L (2018) Peer review of the pesticide risk assessment of the active substance indoxacarb. European Food Saf Authority 16:5140
El-Gendy KS, Radwan MA, Gad AF (2011) Feeding and growth responses of the snail Theba pisana to dietary metal exposure. Arc Environ Contam Toxicol 60:272–280
El-Gendy KS, Radwan MA, Gad AF, Khamis AE, Eshra EH (2019a) Use of multiple endpoints to investigate the ecotoxicological effects of abamectin and thiamethoxam on Theba pisana snails. Ecotoxicol Environ Saf 167:242–249
El-Gendy KS, Radwan MA, Gad AF, Khamis AE, Eshra EH (2019b) Physiological traits of land snails Theba pisana as simple endpoints to assess the exposure to some pollutants. Environ Sci Pollut Res 26:6922–6930
Elia AC, Galarinib R, Dorra AJM, Taticchia MI (2007) Heavy metal contamination and antioxidant response of a freshwater bryozoan (Lophopus crystallinus Pall., Phylactolaemata). Ecotoxicol Environ Saf 66:188–194
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Franeta F, Mirčić D, Todorović D, Milovac Z, Granica N, Obradović S, Perić-Mataruga V (2018) Effects of different insecticides on the antioxidative defense system of the European Corn Borer (Ostrinia nubilalis Hübner) (Lepidoptera: Crambidae) larvae. Arch Biol Sci 70:765–773
Fu H, Xia Y, Chen Y, Xu T, Xu L, Guo Z, Xu H, Xie HQ, Zhao B (2018) Acetylcholinesterase is a potential biomarker for a broad spectrum of organic environmental pollutants. Environ Sci Technol 52(15):8065–8074
Fulton MH, Key V (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 20:37–45
Gaber OA, Elfayoumi HM, El-Shahawy G, Khider FK, Abdel-Tawab H, Mahmoud KA (2022) Influence of Methomyl (Copter 90%) on certain biochemical activities and histological structures of land snails Monacha cartusiana. Saudi J Biol Sci 29:2455–2462
Gamil WE, Mariy FM, Youssef LA, Halim SA (2011) Effect of Indoxacarb on some biological and biochemical aspects of Spodoptera littoralis (Boisd.) larvae. Ann Agric Sci 56:121–126
Ghelichpour M, Mirghaed AT, Hoseinifar SH, Khalili M, Yousefi M, Van Doan H, Perez-Jimenez A (2019) Expression of immune, antioxidant and stress related genes in different organs of common carp exposed to indoxacarb. Aquat Toxicol 208:208–216
Gomot de Vaufleury A (2000) Standardized growth toxicity testing (Cu, Zn, Pb, and pentachlorophenol) with Helix aspersa. Ecotoxicol Environ Saf 46:41–50
Gomot de Vaufleury A, Pihan F (2000) Growing snails used as sentinels to evaluate terrestrial environment contamination by trace elements. Chemosphere 40:275–284
Gunstone T, Cornelisse T, Klein K, Dubey A, Donley N (2021) Pesticides and soil invertebrates: a hazard assessment. Front Environ Sci 9:643847
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. 4. Oxford: Clarendon
Hamed S, Abdelmeguied NE, Essawy AE, Radwan MA, Hegazy AE (2007) Histological and ultrastructural changes induced by two carbamate molluscicides on the digestive gland of Eobania vermiculata. J Biol Sci 7:1017–1037
Hassan HF, Toni ND, Meligi NM (2021) Toxicity induced by indoxacarb exposure in male albino rats and the possible protective effects of vitamin C and zinc. Egypt Acad J Biol Sci C 13:155–176
Hulbert D, Isaacs R, Vandervoot C, Wise JC (2011) Rainfastness and residual activity of insecticides to control Japanese beetle (Coleoptera: Scarabaeidae) in grape. J Econ Entomol 104:1656–1664
Kovačević M, Stjepanović N, Hackenberger DK, Lončarić Ž, Hackenberger BK (2023) Comprehensive study of the effects of strobilurin-based fungicide formulations on Enchytraeus albidus. Ecotoxicology 31:1554–1564
Lapied B, Grolleau F, Sattelle DB (2001) Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels. Br J Pharmacol 132:587–595
Lin Q, Deng P, Feng T, Ou G, Mou L, Zhang Y (2023) Enantioselectivity of indoxacarb enantiomers in Bombyx mori larvae: toxicity, bioaccumulation and biotransformation. Pest Manag Sci 79:2353–2364
Lionetto MG, Caricato R, Calisi A, Giordano ME, Schettino T (2013) Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. BioMed Res Int 2013:321213
Livingstone DR, Garcia-Martinez P, Michel X, Narbonne JF, Hara SCM, Ribera D, Winston GW (1990) Oxyradial production as a pollution-mediated mechanics of toxicity in the common mussel, Mytilus edulis, and other mollusks. Funct Ecol 4:415–424
Louzon M, de Vaufleury A, Capelli N (2023) Ecogenotoxicity assessment with land snails: a mini-review. Mutat Res - Rev Mutat Res 792:108472
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maiza A, Aribi N, Smagghe G, Kilani-Morakchi S, Bendjedid M, Soltani N (2013) Sublethal effects on reproduction and biomarkers by spinosad and indoxacarb in cockroaches Blattella germanica. Bull Insectol 66:11–20
Marigomez IA, Garmendia L, Soto M, Orbea A, Izagirre U, Cajaraville MP (2013) Marine ecosystem health status assessment through integrative biomarker indices: a comparative study after the Prestige oil spill “mussel watch. Ecotoxicology 22:486–505
McLoughlin N, Yin DQ, Maltby L, Wood RM, Yu HX (2000) Evaluation of sensitivity and specificity of two crustacean biochemical biomarkers. Environ Toxicol Chem 19:2085–2092
Mesnage R, Antoniou MN (2018) Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front Public Health 5:361
Monteiro HR, Pestana JLT, Novais SC, Soares AMV, Lemos MFL (2019) Toxicity of the insecticides spinosad and indoxacarb to the non-target aquatic midge Chironomus riparius. Sci Total Environ 666:1283–1291
Orbea A, Fahimi HD, Cajaraville MP (2000) Immunolocalization of four antioxidant enzymes in d of molluscs and crustaceans and in fish liver. Histochem Cell Biol 114:393–404
Owens CWI, Belcher RV (1965) A colorimetric micro-method for the determination of glutathione. Biochem J 94:705–711
Pathak VM, Verma VK, Rawat BS, Kaur B, Babu N, Sharma A, Dewali S, Yadav M, Kumari R, Singh S, Mohapatra A, Pandey V, Rana N, Cunill JM (2022) Current status of pesticide effects on environment, human health and its eco-friendly management as bioremediation: a comprehensive review. Front Microbiol 13:962619
Patra S, Das A, Rakshit R, Choudhury SR, Shyamashree RS, Mondal T, Samanta A, Ganguly P, Alsuhaibani AM, Gaber A, Brestic M, Skalicky M, Hossain A (2022) Persistence and exposure assessment of insecticide indoxacarb residues in vegetables. Front Nutr 9:863519
Peña-Llopis S, Ferrando MD, Peña JB (2002) Impaired glutathione redox status is associated with decreased survival in two organophosphate-poisoned marine bivalves. Chemosphere 47:485–497
Placer ZA, Cushman LL, Jonson BC (1966) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 16:359–364
Prased SN, Muralidhara M (2012) Evidence of acrylamide induced oxidative stress and neurotoxicity in Drosophila melanogaster — its amelioration with spice active enrichment: relevance to neuropathy. Neuro Toxicol 33:1254–1264
Qiao Z, Li P, Tan J, Peng C, Zhang F, Zhang W, Jiang X (2022) Oxidative stress and detoxification mechanisms of earthworms (Eisenia fetida) after exposure to flupyradifurone in a soil-earthworm system. J Environ Manag 322:115989
Radwan MA, Gad AF (2023) Exploring the mechanisms underlying the toxicity of boric acid against the land snail, Theba pisana. Pest Manag Sci 79:1692–1701
Radwan MA, Essawy AE, Abdelmeguied NE, Hamed SS, Ahmed AE (2008) Biochemical and histochemical studies on the digestive gland of Eobania vermiculata snails treated with carbamate pesticides. Pestic Biochem Phys 90:154–167
Radwan MA, El-Gendy KS, Gad AF (2010) Biomarkers of oxidative stress in the land snail, Theba pisana for assessing ecotoxicological effects of urban metal pollution. Chemosphere 79:40–46
Radwan MA, El-Gendy KS, Gad AF (2020) Biomarker responses in terrestrial gastropods exposed to pollutants: a comprehensive review. Chemosphere 257:127218
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117
Regoli F, Gorbi S, Fattorini D, Tedesco S, Notti A, Machella N, Bocchetti R, Benedetti M, Piva F (2006) Use of the land snail Helix aspersa as sentinel organism for monitoring ecotoxicologic effects of urban pollution: an integrated approach. Environ Health Persp 114:63–69
Remia KM, Logaswamy S, Logankumar K, Rajmohan D (2008) Effect of an insecticide (Monocrotophos) on some biochemical constituents of the fish Tilapia mossambica. Pollut Res 27:523–526
Ren B, Jia B, Zhang X, Wang J, Li Y, Liang H, Liang H (2021) Influence of multi-walled carbon nanotubes on enantioselective bioaccumulation and oxidative stress toxicity of indoxacarb in zebrafish (Danio rerio). Chemosphere 267:128872
Rozman KK, Doull J, Hayes WJ (2010) Dose and time determining, and other factors influencing, toxicity. In: Krieger R (ed) Hayes’ Handbook of Pesticide Toxicology, 3rd edn. Academic Press, Cambridge, pp 3–101
Sakthiselvi T, Paramasivam M, Vasanthi D, Bhuvaneswari K (2020) Persistence, dietary and ecological risk assessment of indoxacarb residue in/on tomato and soil using GC–MS. Food Chem 328:127134
Sánchez-Bayo F (2011) Impacts of agricultural pesticides on terrestrial ecosystems, In: F. Sánchez-Bayo, P.J. van den Brink, R.M. Mann (Eds), Ecological Impacts of Toxic Chemicals, Bentham Science Publishers Ltd, Chapter 4, pp 63–87
Sanders T, Schmittmann L, Nascimento-Schulze JC, Melzner F (2018) High calcification costs limit mussel growth at low salinity. Front Mar Sci 5:352
Sbartai I, Berrebbah H, Rouabhi R, Sbartai H, Djebar MR (2012) Induction of oxidative stress in a freshwater ciliated microorganism Paramecium sp., after treatment with indoxacarb. J Biotechnol 6:304–311
Sdeek FA, Taha HS (2018) Indoxacarb residue analysis, dissipation and field efficacy on sugar beet applied for Spodoptera littoralis infestation. Egy Sci J Pestic 4:7–12
Slijkerman DME, Baird DJ, Conrad A, Jak RG, van Straalen NM (2004) Assessing structural and functional plankton responses to carbendazim toxicity. Environ Toxicol Chem 23:455–462
Sogorb MA, Estevez J, Vitanova E (2014) Biomarkers in biomonitoring of xenobiotics (Chapter 57). Gupta RC (ed), Biomarkers in Toxicology, Elsevier, Academic Press, pp. 965–973
Storey KB (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29:1715–1733
Torres MA, Testa CP, Gaspari C, Masutti MB, Panitz CMN, Curi-Pedrosa R, de Almeida EA, Di Mascio P, Filho DW (2002) Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Marine Pollut Bull 44:923–932
U.S. EPA, (2000) Pesticide fact sheet: Indoxacarb. U.S. EPA, Washington, DC
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
van Gestel CAM (2012) Soil ecotoxicology: state of the art and future directions. In: Strus J, Taiti S, Sfenthourakis S (eds), Advances in Terrestrial Isopod Biology, ZooKeys 176: 275–296.
Vasilaki AT, McMillan DC (2017) Lipid peroxidation, in Encyclopedia of Cancer, ed. by Schwab M. Springer, Berlin, Heidelberg
Vasseur P, Cossu-Leguille C (2003) Biomarkers and community indices as complementary tools for environmental safety. Environ Int 28:711–717
Vessey DA, Boyer TD (1984) Differential activation and inhibition of different forms of rat liver glutathione S-transferase by the herbicides 2,4-dichlorophenoxyacetate (2,4-D) and 2,4,5-trichlorophenoxyacetate (2,4,5-T). Toxicol Appl Pharmacol 73:492–499
Widdows J, Nasci C, Fossato VU (1997) Effects of pollution on the scope for growth of mussels (Mytilus galloprovinciallis) from the Venice Lagon, Italy. Mar Environ Res 43:69–79
Wing KD, Sacher M, Kagaya Y, Tsurubuchi Y, Mulderig L, Connair M, Schnee M (2000) Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Prot 19:537–545
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohamed A. Radwan: conceptualization, project administration, supervision, writing — review and editing. Amira F. Gad: investigation, data curation, formal analysis, writing — original draft. Amira M. Abd El-Aziz: investigation, methodology, formal analysis. Kawther S. El-Gendy: validation, visualization, writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval
Research did not involve any human participants and/or animals.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Radwan, M.A., Gad, A.F., Abd El-Aziz, A.M. et al. Does commercial indoxacarb pose ecotoxicological consequences? Employing a multi-marker approach in the model species Theba pisana. Environ Sci Pollut Res 31, 31911–31924 (2024). https://doi.org/10.1007/s11356-024-33214-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33214-z