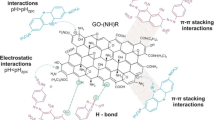
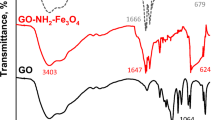
Abstract
Graphene-based adsorbent was prepared by adopting a green synthetic route via the chemical exfoliation of graphite and low-temperature thermal activation. Prepared reactive graphene (RG) was characterized through various techniques, and its adsorption capabilities for textile dye removal were investigated for Acid Blue-93 (AB) and Reactive Red-195 (RR) under different operational conditions. The dye sorption equilibrium and mechanism were comprehensively studied using isotherm and kinetic models and compared statistically to explain the sorption behavior. Results show AB and RR adsorption by RG attains equilibrium in 60 min and 70 min, with a high sorption quantity of 397 mg g−1 and 262 mg g−1 (initial dye concentration of 100 mg L−1), respectively. The dye sorption anticipates that the high surface area (104.52 m2 gm−1) and constructed meso-macroporous features of RG facilitated the interaction between the dye molecules and graphitic skeleton. The R-P isotherm fitted the best of equilibrium data, having the least variance in residuals for both dyes (AB = 0.00031 and RR = 0.00047). The pseudo-second order model best fitted the kinetics of sorption on RG, with chemisorption being the predominant process delimiting step. The overall results promise the dye removal capability of RG to be an efficient adsorbent for azo-based dyes from textile effluents.








Similar content being viewed by others

Data Availability
All the data used in this study is included in the manuscript and supplementary materials.
Abbreviations
- ARE:
-
Average relative error
- AT:
-
Temkin isotherm equilibrium binding constant
- C e :
-
Adsorbate concentrations at equilibrium
- CND:
-
Coefficient of non-determination
- EABS:
-
Sum of absolute errors
- FESEM:
-
Field emission scanning electron microscopy
- FTIR:
-
Fourier transform infrared spectroscopy
- HYBRID:
-
Hybrid fractional error function
- K 2 :
-
Coefficient of non-determination
- K F :
-
Freundlich isotherm constant
- K L :
-
Langmuir affinity constant
- K T :
-
Toth isotherm constant
- MPSD:
-
Marquardt’s percent standard deviation
- n :
-
Freundlich isotherm adsorption intensity
- q e :
-
Sorption capacity at equilibrium
- q max :
-
Maximum adsorption capacity
- R 2 :
-
Coefficient of determination
- RG:
-
Reactivate graphene
- SNE:
-
Sum of Normalized Error
- SSA:
-
Specific surface area
- SSE:
-
Sum of squared errors
- T :
-
Temperature in Kelvin
- UV-Vis:
-
Ultraviolet-visible spectroscopy
- XRD:
-
X-ray diffraction
- n T :
-
Toth isotherm heterogeneity factor
References
Ahmad R (2009) Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J Hazard Mater 171(1):767–773
Alver E, Metin AÜ (2012) Anionic dye removal from aqueous solutions using modified zeolite: adsorption kinetics and isotherm studies. Chem Eng J 200–202:59–67
Ashokan P, Asaithambi M, Sivakumar V, Sivakumar P (2021) Batch and column mode adsorption studies of reactive red 195 dye using Adenanthera paronina L seed activated carbon. Groundw Sustain Dev 15:100671
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276(1):47–52
Bagri A, Mattevi C, Acik M, Chabal YJ, Chhowalla M, Shenoy VB (2010) Structural evolution during the reduction of chemically derived graphene oxide. Nat Chem 2(7):581–587
Belessi V, Romanos G, Boukos N, Lambropoulou D, Trapalis C (2009) Removal of reactive Red 195 from aqueous solutions by adsorption on the surface of TiO2 nanoparticles. J Hazard Mater 170(2):836–844
Biswas S, Sen TK, Yeneneh AM, Meikap BC (2019) Synthesis and characterization of a novel Ca-alginate-biochar composite as efficient zinc (Zn2+) adsorbent: thermodynamics, process design, mass transfer and isotherm modeling. Sep Sci Technol 54(7):1106–1124
Bradder P, Ling SK, Wang S, Liu S (2011) Dye adsorption on layered graphite oxide. J Chem Eng Data 56(1):138–141
Bulin C, Xiong Q, Zheng R, Li C, Ma Y, Guo T (2024) High efficiency removal of methyl blue using phytic acid modified graphene oxide and adsorption mechanism. Spectrochim Acta Part A Mol Biomol Spectrosc 307:123645
Chen C, Zhang X, Deb H, Liang F, Zhu H, Cui K, Zhang Y, Yao J (2021) Synthesis of an eco-friendly bamboo cellulose-grafted-ployacrylamide flocculant and its flocculation performance on papermaking wastewater. Fibers and Polymers 22(6):1518–1525
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Biores Technol 97(9):1061–1085
Deb H, Hasan K, Islam MZ, Kai L, Yang S, Zhang Y, Yao J (2023) The statistical error optimization of dye sorption equilibria for the precise prediction of adsorption isotherms on activated graphene. Applied Sciences 13(14):8106
Deb H, Islam MZ, Ahmed A, Hasan MK, Hossain MK, Hu H, Chen C, Yang S, Zhang Y, Yao J (2022) Kinetics & dynamic studies of dye adsorption by porous graphene nano-adsorbent for facile toxic wastewater remediation. J Water Process Eng 47:102818
Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. J Hazard Mater 167(1):1–9
Deng J-H, Zhang X-R, Zeng G-M, Gong J-L, Niu Q-Y, Liang J (2013) Simultaneous removal of Cd(II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem Eng J 226:189–200
Dey AK, Dey A (2021) Selection of optimal processing condition during removal of Reactive Red 195 by NaOH treated jute fibre using adsorption. Groundw Sustain Dev 12:100522
Doğan M, Alkan M (2003) Adsorption kinetics of methyl violet onto perlite. Chemosphere 50(4):517–528
El-Khaiary MI (2008) Least-squares regression of adsorption equilibrium data: comparing the options. J Hazard Mater 158(1):73–87
Gan L, Li B, Chen Y, Yu B, Chen Z (2019) Green synthesis of reduced graphene oxide using bagasse and its application in dye removal: a waste-to-resource supply chain. Chemosphere 219:148–154
Ganguly A, Sharma S, Papakonstantinou P, Hamilton J (2011) Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J Phys Chem C 115(34):17009–17019
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal – a review. J Environ Manage 90(8):2313–2342
Herbert A, Kumar U (2023) Removal of Acid blue 93 dye from aqueous media using an agro-industrial waste as bioadsorbent: an OPAT and DoE approach. Int J Environ Anal Chem 103(17):5599–5618
Inyinbor AA, Adekola FA, Olatunji GA (2016) Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour Industry 15:14–27
Islam MZ, Dong Y, Khoso NA, Ahmed A, Deb H, Zhu Y, Wentong Y, Fu Y (2019) Continuous dyeing of graphene on cotton fabric: binder-free approach for electromagnetic shielding. Appl Surf Sci 496:143636
Islam MZ, Deb H, Hasan MK, Khoso NA, Hossain MK, Wentong Y, Qi X, Dong Y, Zhu Y, Fu Y (2022) A facile one-pot scalable production of super electromagnetic shielding conductive cotton fabric by hierarchical graphene-composites. J Mater Sci 57(32):15451–15463
Jeong H-K, Lee YP, Lahaye RJWE, Park M-H, An KH, Kim IJ, Yang C-W, Park CY, Ruoff RS, Lee YH (2008) Evidence of graphitic AB stacking order of graphite oxides. J Am Chem Soc 130(4):1362–1366
Jiang H (2011) Chemical preparation of graphene-based nanomaterials and their applications in chemical and biological sensors. Small 7(17):2413–2427
Kamedulski P, Skorupska M, Binkowski P, Arendarska W, Ilnicka A, Lukaszewicz JP (2021) High surface area micro-mesoporous graphene for electrochemical applications. Sci Rep 11(1):22054
Kul AR, Koyuncu H, Turan A, Aldemir A (2023) Comparative research of isotherm, kinetic, and thermodynamic studies for neutral red adsorption by activated carbon prepared from apple peel. Water Air Soil Pollut 234(6):383
Kumar KV, Porkodi K, Rocha F (2008) Isotherms and thermodynamics by linear and non-linear regression analysis for the sorption of methylene blue onto activated carbon: comparison of various error functions. J Hazard Mater 151(2):794–804
Li Y, Du Q, Liu T, Peng X, Wang J, Sun J, Wang Y, Wu S, Wang Z, Xia Y, Xia L (2013) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem Eng Res Des 91(2):361–368
Li Y, Liu Y, Liu Z, Wan X, Chen H, Zhong J, Zhang Y-F (2023) Efficient selective recycle of acid blue 93 by NaOH activated acrolein/chitosan adsorbent via size-matching effect. Carbohyd Polym 301:120314
Li Y, Lu H, Wang Y, Zhao Y, Li X (2019) Efficient removal of methyl blue from aqueous solution by using poly(4-vinylpyridine)–graphene oxide–Fe3O4 magnetic nanocomposites. J Mater Sci 54(10):7603–7616
Lin J, Wang L (2009) Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front Environ Sci Eng China 3(3):320–324
Luhadiya N, Kundalwal SI, Sahu SK (2021) Investigation of hydrogen adsorption behavior of graphene under varied conditions using a novel energy-centered method. Carbon Letters 31(4):655–666
Ma L, Zhao G, Fang Y, Dai W, Ma N (2017) Facile synthesis of mesoporous calcium carbonate particles with finger citron residue as template and their adsorption performances for Congo red. Adsorpt Sci Technol 36(3–4):872–887
Mahmoud ME, Nabil GM, El-Mallah NM, Bassiouny HI, Kumar S, Abdel-Fattah TM (2016) Kinetics, isotherm, and thermodynamic studies of the adsorption of reactive red 195 A dye from water by modified Switchgrass Biochar adsorbent. J Ind Eng Chem 37:156–167
Mahvi AH, Dalvand A (2019) Kinetic and equilibrium studies on the adsorption of Direct Red 23 dye from aqueous solution using montmorillonite nanoclay. Water Quality Res J 55(2):132–144
Menkiti MC, Aniagor CO (2018) Parametric studies on descriptive isotherms for the uptake of crystal violet dye from aqueous solution onto lignin-rich adsorbent. Arab J Sci Eng 43(5):2375–2392
Moreira VR, Lebron YAR, da Silva MM, de Souza SLV, Jacob RS, de Vasconcelos CKB, Viana MM (2020) Graphene oxide in the remediation of norfloxacin from aqueous matrix: simultaneous adsorption and degradation process. Environ Sci Pollut Res 27(27):34513–34528
Nassar MY, Ahmed IS, Mohamed TY, Khatab M (2016) A controlled, template-free, and hydrothermal synthesis route to sphere-like α-Fe2O3 nanostructures for textile dye removal. RSC Adv 6(24):20001–20013
Ng JCY, Cheung WH, McKay G (2002) Equilibrium studies of the sorption of Cu(II) ions onto chitosan. J Colloid Interface Sci 255(1):64–74
Ng JCY, Cheung WH, McKay G (2003) Equilibrium studies for the sorption of lead from effluents using chitosan. Chemosphere 52(6):1021–1030
Pang X-Y, Gong F (2008) Study on the adsorption kinetics of Acid Red 3B on expanded graphite. E-Journal of Chemistry 5:786025
Park S, An J, Potts JR, Velamakanni A, Murali S, Ruoff RS (2011) Hydrazine-reduction of graphite- and graphene oxide. Carbon 49(9):3019–3023
Pérez-Calderón J, Santos MV, Zaritzky N (2018) Reactive RED 195 dye removal using chitosan coacervated particles as bio-sorbent: analysis of kinetics, equilibrium and adsorption mechanisms. J Environ Chem Eng 6(5):6749–6760
Pham T-H, Lee B-K, Kim J (2016) Improved adsorption properties of a nano zeolite adsorbent toward toxic nitrophenols. Process Saf Environ Prot 104:314–322
Pu C, Wan J, Liu E, Yin Y, Li J, Ma Y, Fan J, Hu X (2017) Two-dimensional porous architecture of protonated GCN and reduced graphene oxide via electrostatic self-assembly strategy for high photocatalytic hydrogen evolution under visible light. Appl Surf Sci 399:139–150
Ramesha GK, Vijaya KA, Muralidhara HB, Sampath S (2011) Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J Colloid Interface Sci 361(1):270–277
Sabna V, Thampi SG, Chandrakaran S (2018) Adsorptive removal of cationic and anionic dyes using graphene oxide. Water Sci Technol 78(4):732–742
Salem A-NM, Ahmed MA, El-Shahat MF (2016) Selective adsorption of amaranth dye on Fe3O4/MgO nanoparticles. J Mol Liq 219:780–788
Sarici ÖzdemİrÇ (2018) Adsorptive removal of methylene blue by fruit shell: isotherm studies. Fullerenes, Nanotubes, Carbon Nanostruct 26(9):570–577
Sen GS, Bhattacharyya KG (2011) Kinetics of adsorption of metal ions on inorganic materials: a review. Adv Coll Interface Sci 162(1):39–58
Shao Y, Wang X, Kang Y, Shu Y, Sun Q, Li L (2014) Application of Mn/MCM-41 as an adsorbent to remove methyl blue from aqueous solution. J Colloid Interface Sci 429:25–33
Shi M, Sheng D, Zhuang Q, Xie A, Dong W (2022) Cationically anchored conjugated microporous polymers for fast adsorption of negative dyes from aqueous solution. ACS Appl Polym Mater 4(9):6582–6591
Sreńscek-Nazzal J, Narkiewicz U, Morawski AW, Wróbel RJ, Michalkiewicz B (2015) Comparison of optimized isotherm models and error functions for carbon dioxide adsorption on activated carbon. J Chem Eng Data 60(11):3148–3158
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7):1558–1565
Subbaiah MV, Wen H-Y, Gollakota ARK, Wen J-C, Andrew Lin K-Y, Shu C-M, Mallikarjuna RG, Zyryanov GV, Wen J-H, Tian Z (2022) Removal of sulfonated azo Reactive Red 195 textile dye from liquid phase using surface-modified lychee (Litchi chinensis) peels with quaternary ammonium groups: Adsorption performance, regeneration, and mechanism. J Mol Liq 368:120657
Tan IAW, Ahmad AL, Hameed BH (2008) Adsorption of basic dye using activated carbon prepared from oil palm shell: batch and fixed bed studies. Desalination 225(1):13–28
Touhid SSB, Shawon MRK, Deb H, Khoso NA, Ahmed A, Fu F, Liu XD (2020) Nature inspired rGO-TiO2 micro-flowers on polyester fabric using semi-continuous dyeing method: a binder-free approach towards durable antibacterial performance. Synth Met 261:116298
Vakili M, Rafatullah M, Salamatinia B, Abdullah AZ, Ibrahim MH, Tan KB, Gholami Z, Amouzgar P (2014) Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review. Carbohyd Polym 113:115–130
Wang H, Xie R, Zhang J, Zhao J (2018) Preparation and characterization of distillers’ grain based activated carbon as low cost methylene blue adsorbent: mass transfer and equilibrium modeling. Adv Powder Technol 29(1):27–35
Wong YC, Szeto YS, Cheung WH, McKay G (2004) Adsorption of acid dyes on chitosan—equilibrium isotherm analyses. Process Biochem 39(6):695–704
Wu L, Sun J, Wu M (2017) Modified cellulose membrane prepared from corn stalk for adsorption of methyl blue. Cellulose 24(12):5625–5638
Yan F, Hu D (2019) Insight into the removal of Acid Blue 93 by cationic Eucalyptus cellulose with different alkyl chain lengths: properties and mechanisms. Desalin Water Treat 159:181–192
Yang J, Shojaei S, Shojaei S (2022) Removal of drug and dye from aqueous solutions by graphene oxide: adsorption studies and chemometrics methods. NPJ Clean Water 5(1):5
Zhang F, Yang K, Liu G, Chen Y, Wang M, Li S, Li R (2022) Recent advances on graphene: synthesis, properties and applications. Compos A Appl Sci Manuf 160:107051
Zhang GQ, Bai T, Chen TF, Fan WT, Zhang X (2014) Conversion of methanol to light aromatics on Zn-modified nano-HZSM-5 zeolite catalysts. Ind Eng Chem Res 53(39):14932–14940
Zhang W, Zhou C, Zhou W, Lei A, Zhang Q, Wan Q, Zou B (2011) Fast and considerable adsorption of methylene blue dye onto graphene oxide. Bull Environ Contam Toxicol 87(1):86–90
Acknowledgements
Special thanks are extended to Professor Md. Mizanur Rahman from the School of Mechanical Engineering at Hangzhou Dianzi University, China, for his insightful suggestions and valuable comments that greatly contributed to improving the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. HD and MKH conceptualized and designed the research work. Material preparation, data collection, and analysis were performed by HD, MKH, and MZI. The first draft and revised manuscript was written by HD. SY, YZ, and JY commented on draft and revised manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deb, H., Hasan, M.K., Islam, M.Z. et al. Deep analysis of adsorption isotherm for rapid sorption of Acid Blue 93 and Reactive Red 195 on reactive graphene. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-31918-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-31918-w