Abstract
Microalgae have the potential as a source of biofuels due to their high biomass productivity and ability to grow in a wide range of conditions, including wastewater. This study investigated cultivating two microalgae species, Oocystis pusilla and Chlorococcus infusionum, in wastewater for biodiesel production. Compared to Kühl medium, KC medium resulted in a significant fold increase in cellular dry weight production for both O. pusilla and C. infusionum, with an increase of 1.66 and 1.39, respectively. A concentration of 100% wastewater resulted in the highest growth for O. pusilla, with an increase in biomass and lipid content compared to the KC medium. C. infusionum could not survive in these conditions. For further increase in biomass and lipid yield of O. pusilla, different total dissolved solids (TDS) levels were used. Maximum biomass and lipid productivities were achieved at 3000 ppm TDS, resulting in a 28% increase in biomass (2.50 g/L) and a 158% increase in lipid yield (536.88 mg/g) compared to KC medium. The fatty acid profile of O. pusilla cultivated on aerated wastewater at 3000 ppm TDS showed a high proportion of desirable saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) for biodiesel production. Cultivating microalgae in wastewater for biodiesel production can be cost-effective, especially for microalgae adapted to harsh conditions. It could be concluded that O. pusilla is a promising candidate for biodiesel production using wastewater as a growth medium, as it has high biomass productivity and lipid yield, and its fatty acid profile meets the standard values of American and European biodiesel standards. This approach offers a sustainable and environmentally friendly solution for producing biofuels while reducing the environmental impact of wastewater disposal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well-known that the utilization of conventional fuels in energy generation has widely acknowledgedly led to a range of environmental issues, including air pollution, the release of greenhouse gases, and the onset of climate change. Fossil fuels are finite resources that are being depleted rapidly, and their extraction and use significantly impact local communities and ecosystems. In addition, the geopolitical implications of fossil fuel dependence have led to conflicts and geopolitical instability (Abdelsalam et al. 2019; Abomohra and Elshobary 2019). Therefore, there is an immediate requirement to shift towards renewable and sustainable energy sources to mitigate these environmental and social issues. Microalgae-based biodiesel production has the potential to be a sustainable and eco-friendly alternative to conventional fuels, as it can be produced using renewable resources such as sunlight and wastewater and has a lower carbon footprint compared to fossil fuels (EL-Seesy et al. 2021; Chandrasekhar et al. 2022). By promoting the development of biomass-based biodiesel production, we can take a step towards reducing our reliance on conventional fuels and addressing the environmental and social problems associated with their use.
Algae have gained significant attention as a potential in everything from food, feed, bio-energy, pharmaceutical, and cosmetics (El‐Khodary et al. 2020; Siddiki et al. 2022; Huo et al. 2022; El-Sapagh et al. 2023). Because of their substantial lipid content, swift growth rate, and ability to flourish in various settings, microalgae have garnered significant attention as a feasible option for biodiesel production (Venkata Subhash et al. 2022; Ali et al. 2023). The high cost of microalgae cultivation and the low biomass productivity are two significant obstacles to commercializing microalgae-based biodiesel production. Cultivating microalgae requires substantial resource allocation, including water, nutrients, and energy, which can incur significant costs.
An encouraging strategy to cut down on the expenses associated with microalgae cultivation involves employing wastewater as a growth medium. Wastewater is a cheap and abundant resource that can provide nutrients and water for microalgae growth. In addition, using wastewater for microalgae cultivation can help to reduce environmental pollution by treating the wastewater and recovering valuable resources (Elshobary et al. 2019; Wang et al. 2023a, b). Numerous studies have consistently demonstrated that the utilization of wastewater yields not only the benefit of wastewater recycling but also presents a significant opportunity for biomass conversion and production. This phenomenon is markedly pronounced in the context of microalgae-based biofuel production and various other applications, all of which hold exceptional promise. Microalgae cultivated in wastewater can amass lipids, varying from minimal levels at 10% of dry weight to more substantial amounts at 25–30% of dry weight, accounting for > 60% of their dry weight (Abou-Shanab et al. 2013). Algae, owing to their lipid accumulation capacity and robust photosynthetic productivity, exhibit a yearly oil yield that surpasses palm oil by a significant margin, ranging from 7 to 31 times higher (Hu et al. 2008; El-Sheekh et al. 2017). Biodiesel derived from microalgae represents a potential sustainable energy source capable of substituting fossil fuels, all while safeguarding the availability of food resources for human consumption (Enamala et al. 2018; Elshobary et al. 2022). While microalgae have demonstrated potential as a feedstock for biodiesel manufacture, the challenges of selecting a suitable microalgal species, enhancing growth rate, and stimulating lipid accumulation still exist. Choosing an appropriate algal strain is paramount for efficient biodiesel production, as different strains vary in their lipid content, growth rate, and tolerance to environmental stressors. Enhancing the growth rate of microalgae can also be challenging, as it requires optimizing various growth factors such as temperature, pH, light intensity, and nutrient availability. Additionally, motivating cellular lipid synthesis and accumulation in algae requires the application of specific stressors or genetic modifications to alter the metabolic pathways of algae (Barakat et al. 2021). Attempts are underway to address these encounters and develop more efficient and sustainable microalgae-based biodiesel production methods. For example, mutation techniques can modify microalgae's metabolic pathways to increase their lipid content and enhance their growth rate (Elshobary et al. 2022). Additionally, using low-cost and sustainable growth media such as wastewater and developing efficient lipid extraction methods can help lower the total cost of biodiesel production from microalgae (Abomohra and Elshobary 2019; Wang et al. 2023b). While the high costs of cultivation and extraction have been a major barrier, recent techno-economic analyses suggest the potential for commercially viable microalgae biodiesel production through optimized processes and scaled-up systems to reduce costs (Xin et al. 2016).
To further enhance the lipid content in microalgae, various stress environments such as nutrient deficit (Pandit et al. 2017) as well as salinity stress (measured by total dissolved solids or TDS) can be applied during cultivation. The application of salinity stress to enhance lipid production in microalgae has been broadly investigated and applied in the production of biodiesel (Paliwal et al. 2017; Abomohra et al. 2020) for freshwater microalgae such as Chlorella sp. (Yun et al. 2019) or even marine species Dunaliella (BenMoussa-Dahmen et al. 2016), Tetraselmis sp. and Nannochloropsis sp. (Khatoon et al. 2014). Salinity stress is a common environmental stressor that can be easily controlled during microalgae cultivation. It involves exposing microalgae to high salt concentrations, which can alter their physiology and metabolism, leading to the accumulation of lipids. In this regard, microalgae have evolved distinct mechanisms to adjust to salinity stress. These mechanisms may include the accumulation of osmo-protective solutes, the production of antioxidant enzymes, the regulation of ion exchange processes, and a shift from active cell division to energy storage in the form of lipids (Talebi et al. 2013). The degree of lipid accumulation varies depending on the salinity levels and the microalgae species. Salinity stress could potentially be problematic for certain types of microalgae, given that each strain has a unique ideal salinity level for both growth and lipid accumulation. Thus, the examination of microalgae to identify a species with high lipid content across a broad range of salinity levels is crucial. While some studies have explored microalgae cultivation on wastewater, there is a limited understanding of how different microalgae species respond to 100% wastewater and increasing salinity levels (Elshobary et al. 2019). This study aimed to fill this knowledge gap by evaluating the biomass and lipid productivity potential of two new native strains that are naturally adapted to grow in the wastewater environment available locally, microalgae species, Oocystis pusilla and Chlorococcus infusionum, grown on 100% wastewater and with varying total dissolved solids (TDS) levels. We demonstrate the feasibility of using wastewater to unlock the potential of O. pusilla for biodiesel production through significantly increased biomass and lipid yields. This innovative approach could provide a cost-effective and environmentally sustainable solution for microalgal biofuel production.
Materials and methods
Microalgae isolation
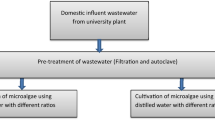
Two microalgae species were isolated from the aerated bond muncible wastewater (AWW) treatment plant located in ESH ELMALAHA of Hurghada, Egypt (25°04′10.0″N 34°53′15.2″E). The wastewater samples were transported to the laboratory and cultured into a 10% Kühl medium (Kühl and Lorenzen 1964) at room temperature (25 ± 3 °C) for a week under continuous illumination of cool fluorescent lamps (40 µmol m−2s−1; Philips Co., Taipei, Taiwan). Following a visual assessment of algal growth, 20 μL of the culture was streaked onto a solid Kühl medium containing 2% agar. To acquire pure cultures, the green algae were isolated and purified through the sub-culturing technique. The microalgae cells were examined microscopically (Olympus BH2, Olympus, Shinjuku-ku, Tokyo, Japan). Algal morphological characters were examined, and images of algal structures were captured, encompassing their form, dimensions, and cellular features such as the flagella, chloroplast, and cell wall. These isolated microalgae were classified by referring to previously documented findings (Smith 1950; Hendy 1964; Prescott 1987). The isolated algae were conserved on Kühl (1.5%) medium agar slants at 4 °C for future work.
Wastewater characterization
Water samples were collected from the aerated bond wastewater (AWW) treatment plant located in ESH ELMALAHA of Hurghada, Egypt, at geographic coordinates 25°04′10.0″N 34°53′15.2″E. In situ wastewater temperature, electrical conductivity, and pH measurements were monitored using the Hydrolab Model (Multi Set 430i WTW). Secchi-disk was used to determine transparency (diameter 30 cm). Alkalinity, chemical oxygen demand (COD), total suspended solids (TSS), dissolved oxygen (DO), biochemical oxygen demand (BOD), and chemical oxygen demand (COD) were performed according to Winkler’s method (Strickland and Parsons 1972). Nutrients including chlorides (CL−), nitrite (NO2−2-N), nitrate (NO3−1-N), ammonium (NH4+-N), total nitrogen (TN), total phosphorus (TP), total dissolved solids (TDS), sulfides(S−2), and total hardness (TH) were measured in accordance with the standard procedures established by the American Public Health Association (APHA 2005).
Microalgae cultivation
Two photoautotrophic media were used, Kühl medium and KC (Kessler and Czygan 1970), for microalgae cultivation (Table 1). The microalgal cultures were incubated at 25 ± 2 °C under a constant fluorescent light of 40 µmolm−2 s−1 and were constantly supplied with sterilized filtered air to prevent biomass from glomming at the flask’s bottom. The experiments were conducted in triplicate using 1000 mL Erlenmeyer flasks filled with 500 mL of culture medium and each inoculated with 10% of a vigorously growing microalgal culture.
Cultivation of microalgae in wastewater mixed with synthetic medium
Areated wastewater (AWW) samples were collected and settled for 3 h at room temperature and the supernatant was used after sterilization in algal cultivation dilutions. The two isolated microalgae were grown in undiluted (100%) and diluted (25%, 50%, and 75%) AWW. Dilutions were made by adding the required amounts of AWW and KC medium. The experiments were conducted in triplicate in 2 L Erlenmeyer conta filled with 1 L of culture medium (each flask was stopped with cotton plugs and sterilized in autoclave at 121 ℃,1.5 bar for 20 min) after sterilization and cooling culture medium inoculated with 10% of exponentially growing microalgal culture. Each dilution was cultivated under the same cultivation condition of the “Microalgae cultivation” section. Microalgae grown in KC medium under the same growth conditions were sustained as the control.
Studying the effect of different salt stress on algal performance
For improving the algal growth and lipid productivity, different NaCl concentrations ranging from 1.73 to 3.23 g/L were employed to modify the TDS levels from 2000 to 3500 mg/L. TDS levels of the modified medium were determined using a HACH TDS meter (model: sensION™ + EC71).
Growth measurements
Algal growth was determined by optical density (OD) at 680 nm (Markle et al. 2000), at two days intervals using SHIMADZU UV-2401PC, Japan spectrophotometer. Dry weight (DW) was monitored by gravimetry. A linear correlation between OD680 and dry weight was determined for both algal strains:
Biomass productivity was calculated according to Eq. (3)
where, DWE (mg/L) and DWi (mg/L) represent the algal dry weight at the initial and the end of exponential growth phase, respectively, while ΔT is the time difference.
Estimation of lipid content for the isolated species
The lipid content was extracted by mixing a 100 mg of algal pellet with 20 mL chloroform: methanol (2:1, v/v) (Folch) and incubating overnight at 25 ± 2 °C in a shaking incubator. The mixture was subjected to centrifugation at 1500 × g for 10 min. Following centrifugation, the supernatant was carefully collected, and the remaining residue underwent two additional extraction cycles. The solvent was mixed with 2 mL of 1M NaCl. The lipid phase was collected and left to evaporate, and the total lipid content was estimated gravimetrically. Lipid productivity (LP) was calculated using Eq. (4):
where LCE and LCi are the lipids content at the ending and initial of the exponential phase, respectively, and ΔT is the time difference.
Fatty acid analysis
Next to lipid extraction, the transesterification method was carried out according to Radwan (1978) in which 50 mg of lipid was mixed with 5 mL of methanolic sulphuric acid mix (1:100 v/v sulphuric acid (98%) and methanol (100%) and 2 mL of benzene. The mix was heated in the water bath (WB) for 90 min at 90 ℃. Once cooled, the procedure involved adding 8 mL of distilled water and 5 mL of petroleum ether to the mixture, followed by thorough mixing. The ethereal oil layer containing fatty acid methyl esters (FAME) was separated and evaporated. This resulting material was utilized for gas–liquid chromatography. FAMEs were resuspended in 20 µL of acetonitrile and subjected to analysis using an HP-6890 gas–liquid chromatograph equipped with an HP-5 column (30m × 0.32 mm × 0.25 µm) and a flame ionization detector (FID). The temperature program for the chromatograph began at 140 °C for 3 min, followed by an increase at a rate of 1.5 °C per minute until it reached 187 °C. Subsequently, the temperature was raised at a rate of 5 °C per minute until it reached 220 °C, and this temperature was maintained for 5 min. The detector operated at 250 °C, and the injector temperature was set at 220 °C. The injection was performed in a splitless mode using one µL of FAMEs, and the carrier gas (nitrogen) flowed constantly at a rate of 1 mL/ min.
The assessment of fuel properties for the generated algal fatty acid profiles
The assessment of fuel properties for the generated algal fatty acid profiles involved theoretical calculations of various biodiesel properties. These properties encompassed the average degree of unsaturation (ADU %), cetane number (CN), iodine value (IV, g I2.100/g oil), kinematic viscosity (υi, mm2/s), specific gravity (ρ), cloud point (CP, ℃), saponification value (SV, mg KOH/g), long chain saturation factor (LCSF, wt %), higher heating value (HHV), and cold filter plugging point (CFPP, °C). These calculations were performed using Eqs. (5) through (13), as described in studies by
where N represents the number of carbon–carbon double bonds in each fatty acid, and Mf is the mass fraction of each fatty acid.
where C16:0, C18:0, C20:0, C22:0, and C24:0 is the percentage of the subsequent fatty acids.
where X represnts the content of linoleic and linolenic acids (wt %) (0 < X < 100), and Y is the oxidation stability per hour.
Statistical analyses
The results are presented as the mean of three replicates, along with the standard deviation (SD) indicated as ± . To determine the significance of the data, a statistical analysis was conducted using a one-way analysis of variance (ANOVA) with a significance level set at p ≤ 0.05. The statistical analyses were performed using the SPSS software (IBM, version 22), and Duncan’s multiple range tests were applied for post hoc analysis. This rigorous statistical approach helps to assess and compare the data effectively.
Results and discussion
Water characteristics
The characteristics of the municipal wastewater used as a cultivation medium are detailed in Table 2. The wastewater had a moderate pH of 7.35 and a temperature of 26.54 °C. Dissolved oxygen was low at 1.05 ppm, while BOD and COD levels were elevated at 179.23 and 304.66 ppm, respectively. Turbidity was 9.67 NTU, with total dissolved solids (TDS) of 1500 ppm and total suspended solids (TSS) at 195 ppm. The wastewater was high in chlorides (1520.69 ppm) and alkalinity (600.72 ppm). Nutrient levels included sulfur compounds at 2.05 ppm, nitrite at 36.25 ppm, nitrate at 6.02 ppm, and phosphate at 47.02 ppm. This composition provided nutrients to support algal growth while requiring acclimation to the elevated organic matter. Municipal wastewater, also known as domestic wastewater, is the wastewater discharged from households, including bathrooms, laundry rooms, and kitchens. When compared to various other types of wastewater, municipal wastewater contains relatively lower content of nitrogen (ranging from 15 to 90 mg/L), phosphorus (ranging from 5 to 50 mg/L), and archetypally exhibits a reduced concentration of chemical oxygen demand (approximately 300 mg/L) (You et al. 2022). Due to these characteristics, municipal wastewater is often considered suitable for the cultivation of microalgae.
Microalgae identification
Our primary objective is to uncover and characterize novel indigenous microalgal strains that have naturally adapted to thrive in the specific wastewater conditions present in the local environment. By systematically screening microalgae from the target waste stream, we can pinpoint species that have undergone optimization processes specifically tailored to this unique ecological niche. Moreover, the use of native strains alleviates concerns related to the potential introduction of invasive foreign species into the ecosystem. In the course of this study, we successfully isolated and identified two native microalgae strains. These identifications were primarily based on morphological characteristics. The isolated microalgae have been identified as follows: Oocystis pusilla Hansgirg 1890: This species exhibits a colonial, oval form, with sizes ranging from 15 to 30 µm Fig. S1, A and Chlorococcus infusionum (Turpin) Kützing 1833: Characterized by its single-cell structure, this species takes on a spherical shape, typically measuring between 15 and 40 µm Fig. S1, B. Both of these species belong to the Chlorophyta phylum.
This strategic focus on native microalgae strains enhances our ability to harness the unique adaptability and resilience of these organisms, which have evolved to thrive in the specific wastewater conditions found within our local ecosystem.
Determination of the best growing medium
The choice of an appropriate growth medium for superior biomass and lipid production is based on the algae’s growth needs, the impact of the medium’s components on the concluding product quality, and the cost of the medium’s components (Abomohra et al. 2016). KC and Kühl media were used for the cultivation of the isolated microalgae. KC medium showed better algal growth relative to Kühl medium. The KC medium is suitable for the growth of both algae as it contains low nitrate and NaH2PO4.H2O content and higher levels of Na2HPO4.2H2O, MgSO4.7H2O, and (NH4)6Mo7O24.4H2O as sources of phosphorus, magnesium, and molybdenum, respectively, compared to other growth media. However, the presence of NaCl in the KC medium can lead to alterations in water salinity, which, in turn, may have notable effects on the growth, metabolism, and photosynthesis of microalgae. The salt concentration in the culture medium is a critical factor to consider when studying microalgal physiology and biochemistry, as it can influence various cellular processes and ultimately impact research outcomes (Abomohra et al. 2020).
On the other hand, C. infusionum showed low cell density compared to O. pusilla (Fig. 1A). The overall higher OD values achieved by O. pusilla compared to C. infusionum correlate with its higher biomass reported in Fig. 1B. The timing of the OD decrease varies between species and media types, likely reflecting differences in nutrient availability and uptake kinetics. KC medium supported a later peak and decline for O. pusilla, possibly due to more controlled nutrient release from the defined composition. The earlier peak and crash in KC cultures by C. infusionum suggests faster nutrient depletion in this complex medium, which may be due to the higher metabolism of C. infusionum rapidly exhausting nutrients in KC. It was observed that O. pusilla has a relatively long exponential phase compared to C. infusionum, extending to the 20th and 24th day in KC and Kühl media, respectively. This may be related to the nature and form of each species since colonial O. pusilla may exhibit different growth patterns compared to single-celled C. infusionum due to factors like cell–cell interactions, nutrient uptake, and light utilization. The shared sheath of O. pusilla may provide benefits like retaining nutrients and metabolites. The peak algal dry weight (1.73 g/L) was recorded in O. pusilla cultivated on KC medium on the 20th day, while only 1.047 g/L on Kühl medium on the 24th day in the stationary phase. The same trend was recorded in lipid content, where the most lipid content (224.17 mg/g) was demonstrated in O. pusilla cultivated on KC medium (Fig. 1B). Accordingly, KC medium was selected for the next experiment.
The growth phase of algal culture can affect the fatty acids profiles and lipid content. In some species, lipid content improves during the stationary growth phase, including O. pusilla and C. infusionum. The observed changes in microalgal physiology and lipid content in could be attributed to a shift in lipid metabolism. This shift may involve transitioning from synthesizing membrane lipids to storing neutral lipids. This metabolic change is likely prompted by the depletion of crucial nutrients, such as nitrogenous compounds required for protein synthesis and phosphate-containing compounds necessary for the formation of phospholipids, which are key structural components of cell membranes. As a result, the microalgae may prioritize the accumulation of neutral lipids, potentially for energy storage or other metabolic purposes, in response to the nutrient limitations induced by the salinity changes. (Xu et al. 2020; Chen and Wang 2021). Bigogno (2002) also found a similar increase in total fatty acid content and triacylglycerols in Parietochloris incise during the stationary phase, from 43% in the logarithmic phase to 77%.
Biomass and lipid production in the wastewater mixture
Different concentrations (0, 25, 50, 75, and 100%) of KC medium were used mixed with AWW to cultivate both isolates. The tested microalgae showed different patterns regarding the ability to grow in AWW (Fig. 2). Results showed that O. pusilla was more adapted to grow in AWW than C. infusionum. In O. pusilla, 100% AWW (0%KC) led the maximum cell density reaching 4 OD (Fig. 2A) with a dry weight of 1.96 g/L at the 20th day of cultivation (Table 3), while C. infusionum was seriously affected by wastewater, and the highest cell density of 2 was recorded in 100% KC (Fig. 2B) with 1.05 g /L and decreased by increasing AWW concentration (Table 3). On the other hand, growing O. pusilla on 0% AWW (100% KC control) represents in the second order, where the highest OD and biomass were 3.35 and 1.73 g/L. When the KC medium was diluted with AWW, O. pusilla’s growth decreased, showing moderate growth in a medium composed of 25% AWW and 75% KC medium. This condition led to moderate biomass concentrations over 15 days of cultivation, followed by a decline in density after 15 days (Fig. 2A). The KC medium likely provided essential nutrients and optimized conditions that initially supported O. pusilla’s growth. However, the diluted KC medium reduced growth slightly at 25% AWW compared to 100% KC medium.
The growth of O. pusilla showed a decreasing trend initially as the wastewater concentration increased from 25 to 50%, but then increased with further increases in wastewater concentration up to 100% (Fig. 2A). The initial decline in growth in the 50% wastewater experiment compared to 25% wastewater might be due to the low optimum nutrient levels and the different components of wastewater versus the nutrient level at the 25% experiment. However, in experiments with 75% and 100% wastewater, O. pusilla likely preferred the wastewater conditions of low nitrate and phosphate content compared to the KC medium, allowing it to thrive and achieve higher growth. The increasing growth with higher wastewater concentrations in individual experiments indicates O. pusilla’s ability to utilize low mixed nutrient loads such as nitrite, nitrate, and phosphate, a useful trait for cultivation using wastewater as a growth medium.
A similar tendency was detected in terms of lipid content, with the highest biomass also showing the highest lipid content. The most significant lipid accumulation of 303.42 mg/g dw was observed in O. pusilla cultivated in 100% AWW, while C. infusionum showed a maximum lipid accumulation of 224.17 mg/g dw in 100% KC medium (Table 3). O. pusilla demonstrated a remarkable adaptability to highly concentrated municipal wastewater.
Several investigations have demonstrated that microalgae have the capacity to effectively utilize phosphorus, nitrogen, and organic substances from different wastewater sources. This process not only helps in the purification of wastewater but also leads to the generation of biomass. These wastewater sources encompass municipal, agro-industrial, industrial, and livestock wastewater (Srinuanpan et al. 2020; Srimongkol et al. 2022). For example, Tribonema minus has been found to thrive in simulated butyric acid wastewater and its biomass and lipid productivity accomplished the superior of 223.80 and 108.16 mg/L/day, respectively (Wang et al. 2023a, b), while halophilic oleaginous microalgae Nannochloropsis oculata and Tetraselmis chuii have demonstrated survival in industrial wastewater and the highest biomass and lipid productivity were documented using a ratio of 75% industrial WW:25% F/2 medium (Essa et al. 2018). Additionally, Chlorella pyrenoidosa has been shown to survive in piggery wastewater (Wang et al. 2012). A study by Shu et al. compared the growth and lipid content of three microalgae species (Chlorella zofingiensis, Chlorella sp., and Scenedesmus sp) in BG11 medium and raw dairy wastewater. The study found that all three microalgae species grew faster and produced more lipids in dairy wastewater than in synthetic medium (Shu et al. 2018). Another study compared the growth and lipid yield of three green microalgae, Scenedesmus obliquus Chlamydomonas reinhardtii, and Monoraphidium braunii, cultivated in sewage water and synthetic medium. It was observed that the growth rates of both S. obliquus and M. braunii were substantially reduced when cultivated in wastewater compared to the control group cultivated in synthetic medium. In contrast, C. reinhardtii displayed the most significant growth rate when grown in sewage wastewater (El-Sheekh et al. 2023).
Conversely, the lipid contents and productivity of C. reinhardtii, M. braunii, and S. obliquus were enhanced when cultivated in sewage wastewater as opposed to synthetic medium (El-Sheekh et al. 2023). This indicates that different microalgae species may have varying responses to wastewater conditions. As demonstrated in this study, O. pusilla appears to excel in wastewater cultivation compared to C. infusionum. This variability in behavior between these two microalgae species can be attributed to their distinctive abilities, not only to survive and flourish in wastewater but also to effectively harness its nutrients. These differences in adaptation and nutrient utilization strategies make certain microalgae, like O. pusilla, well-suited for wastewater-based cultivation, presenting valuable opportunities for applications in biofuel production.
The lipid productivities achieved by O. pusilla and C. infusionum are comparable to those reported for other high-oil-producing microalgal species grown on wastewater. A recent study isolating oleaginous Oocystis sp. and Chlorococcum sp. from dairy farm wastewater found Oocystis sp. had superior lipid content (24.70% dw) compared to Chlorococcum sp (18.68% dw) (Sun et al. 2018). The elevated lipid content observed in O. pusilla cultivated in 100% AWW (secondary treated municipal wastewater) may be attributed to variations in biotic stress responses among different algal species. Additionally, it could result from a notable deficiency in essential elements, particularly nitrogen, within the secondary treated municipal wastewater. These conditions might have triggered a metabolic response in O. pusilla that prioritizes lipid accumulation, possibly as an adaptive strategy to cope with nutrient limitations and environmental stressors present in the wastewater. This nitrogen scarcity led to an increased accumulation of lipids (Rodríguez-Palacio et al. 2022). These findings are consistent with prior research by Elshobary et al. (2019) and El Shafay et al. (2021), who demonstrated that green microalgae (Micractinium reisseri) and blue-green algae (Anabaena variabilis and Nostoc muscorum) tend to amass superior lipid content when subjected to nitrogen-low conditions.
However, C. infusionum cannot survive in such highly polluted conditions in 100% AWW. This result could be explained by C. infusionum having different nutrient requirements or metabolic pathways compared to O. pusilla, allowing O. pusilla to achieve better biomass and lipid yields. The nutrient content, salt, or other constituent concentrations in the 100% wastewater may have exceeded the tolerance levels of C. infusionum, inhibiting its growth. O. pusilla appears more tolerant of harsh wastewater conditions. The difference in behavior of both microalgae is attributed to the ability of each species to survive and grow in wastewater as well as utilize its nutrients efficiently (Sforza et al. 2020). As a result, O. pusilla cultivated in 100% AWW was used for the remainder of the study.
Effect of salt stress on the growth and lipid content of O. pusilla
Four concentrations of NaCl were used to study their impact on the growth performance and lipid production of O. pusilla. Figure 3 shows that NaCl at a TDS concentration of 3000 ppm showed the maximum biomass and lipid content of 2.5 g/L and 536.88 g/L, with an improvement of 27.55% and 76.94% compared to the control (1500 ppm). Salinity stress has yielded lipid storage in microalgae (Venkata Mohan and Devi 2014; El-Sheekh et al. 2019). When exposed to salinity stress, microalgae undergo various physiological changes to adapt to their new environment. One of these changes is an improvement in lipid accumulation. This is because lipids play an essential role in cellular membrane composition and function, and high salt concentrations can disrupt the integrity of the membrane. As a result, the microalgae increase their lipid production to repair and protect their cellular membranes. When biomass and lipid productivities were studied, yields showed higher values in the salinity condition of 3000 ppm (113.33 and 52 mg/L/d, respectively) (p < 0.01) because of higher growth and higher lipid content (when compared to other TDS concentrations) (Table 4). Assuming high biomass and lipid productivities are desired for the utilized biofuel production, TDS in the upper level at 3000 ppm of high growth of O. pusilla would maximize lipid production. The hypersaline levels at 3000 ppm of O. pusilla grown on 100% AWW could likely enhance O. pusilla’s fatty acid profile and transesterification to biodiesel.
Fatty acid profile O. pusilla
The fatty acid content analysis for O. pusilla cultivated in 100% AWW with a total dissolved solids (TDS) of 3000 ppm, where the highest biomass and lipid productivity were observed, revealed some interesting findings. In Table 5, it is shown that the saturated fatty acid (SFA) content was notably high, at 41.76%, followed by monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA).
Among the detected fatty acids with chain lengths ranging from C12 to C18, C16:0 stood out as the dominant saturated fatty acid, constituting 30.38% of the total fatty acids. On the other hand, C18:1 was the predominant fatty acid in the monounsaturated category, while C18:2 was the dominant one in the polyunsaturated category (Table 5).
Interestingly, O. pusilla primarily produced fatty acids with chain lengths of C16 and C18, accounting for a significant portion, specifically 73.87%, of the total fatty acid methyl esters (FAME). Furthermore, the algae displayed low levels of unsaturation in their fatty acids, and no polyunsaturated fatty acids with four or more double bonds were detected. This fatty acid composition and structure are considered favorable for biodiesel production, indicating the potential suitability of O. pusilla as a feedstock for biodiesel production due to its high content of saturated and monounsaturated fatty acids.
The findings from the current study align with those of Matos et al. (2015) and Abomohra et al. (2020). Their research explored the impact of desalination concentrate on the fatty acid profile of N. gaditana and Dunaliella salina KSA-HS022, respectively. They observed a similar trend, where an increase in the concentration of desalination concentrate led to an increase in saturated fatty acids (SFAs) at the expense of polyunsaturated fatty acids (PUFAs). This consistency in results across different studies underscores the significance of environmental factors, such as salinity or nutrient availability, in influencing the fatty acid composition of microalgae.
Biodiesel quality
This study calculated and compared the essential qualitative characteristics of biodiesel produced from each treatment to the standards outlined in the American (ASTM D6751) and European (EN 14214) international guidelines, as illustrated in Table 6. The results indicate that the kinematic viscosity (υi), cetane number (CN), iodine value (IV), cloud point (CP), specific gravity, and higher heating value (HHV) values met the criteria set by both ASTM D6751 and EN14214 standards.
Furthermore, the average degree of unsaturation (ADU) of O. pusilla FAMEs was determined to be 0.55, which indicates a relatively low level of unsaturation. It is well-established that a low degree of unsaturation is a crucial factor in determining the quality of biodiesel. This ADU value is consistent with various ADU values observed in biodiesel derived from different microalgae sources, suggesting that O. pusilla-derived biodiesel aligns with the established standards and quality characteristics of biodiesel in this context. (Ashour et al. 2019; Elshobary et al. 2019, 2022; El Shafay et al. 2021) or macroalgae (Elshobary et al. 2020; Osman et al. 2020). The CN measures the ignition efficiency of biodiesel, increasing as the saturation level of fatty acids increases (Karpagam et al. 2015). Saturated long-chain esters such as methyl palmitate C17H34O2 and methyl stearate C19H38O2 have high cetane numbers (Knothe 2016). O. pusilla obtained biodiesel rich in these two fatty acids methyl esters 37.05%. Following international standards such as ASTM D6751-02, which recommends a minimum cetane number (CN) of 47, and EN14214, which suggests a CN of at least 51, it was found that the biodiesel produced by O. pusilla exhibited a favorable CN value of 59.2. This value ensures that the engine can initiate combustion quickly and quietly, indicating efficient ignition performance and minimal emissions of nitrogen oxides (NOx) (Ashokkumar et al. 2015; Sharma et al. 2016). It is important to note that the lowest IV value indicates the highest saturation level of methyl esters and offers high stability against oxidation (Srinuanpan et al. 2018), in contrast to the CN value. Our results were steady with different biodiesels produced from diverse green microalgae (Wang et al. 2013, 2023b; Song et al. 2013; Elshobary et al. 2019). Biodiesel generally has a higher cloud point compared to petroleum diesel fuel. In the current study, the cloud point (CP) of O. pusilla was 12.63 °C, which enhances its suitability for use in low-temperature conditions. Similarly, the recommended biodiesel strain, Tribonema minus, also showed 12.50 °C cloud point values when cultivated on synthetic butyric acid wastewater (Wang et al. 2023b). The higher heating value (HHV) refers to the quantity of heat generated by completely burning a specific amount of fuel. According to previous studies, the HHV values obtained in the current study were deemed satisfactory (Zhang et al. 2018; Elshobary et al. 2022) that is higher than that recorded in Oocystis submarina ( 29.47 and 32.98 MJ kg−1) under 100% and 50% of F/2 synthetic medium, respectively (Hawrot-Paw et al. 2021). This variation could be due to differences in the fatty acid composition of both species and the growth conditions, as algae have the ability to alter their fatty acid profile as well as morpho-anatomical and biochemical characterization in facing environmental stress (Barakat et al. 2021; Senousy et al. 2023; Ismail et al. 2023). Another parameter calculated was LCSF, which determines the fuel’s cold flow properties and is usually detected by the cold filter plugging point (CFPP). The information in Table 5 clearly shows O. pusilla’s long chain saturation factor (LCSF) value, which was determined to be 6.37. Additionally, the calculated cold filter plugging point (CFPP) value was found to be 3.55 °C. It is worth noting that CFPP values can vary depending on the level of unsaturation in the methyl ester. As per the findings of Ramos et al., lower CFPP values are preferred, and this study recorded a CFPP value of 3.55 °C, which is in line with the desirable characteristic of having a lower CFPP for biodiesel (Ramos et al. 2009) and falls within the EN14214 range (≤ 5 ≥ –20 °C). The oxidation stability of the biodiesel produced from O. pusilla, which indicates the formation of sediments, resins, and acids in the fuel, was found to be 11 h. This result meets the European standard (EN14214).
Based on these findings, it can be concluded that the cultivation of O. pusilla in the Hurghada aerated wastewater plant resulted in biodiesel that complies with both American standards (ASTM D6751-02) and European standards (EN 14214) in all key properties. This indicates the potential suitability of O. pusilla-derived biodiesel for use in accordance with these international standards.
Implementation feasibility assessment
While this study does not provide an extensive feasibility analysis demonstrating the practical applications, it is possible to derive a reasonable assessment of the potential benefits of utilizing wastewater effluent as a growth medium for O. pusilla by incorporating data from this study. The results were specifically utilized to illustrate the impact of wastewater utilization on the cost of microalgal biomass production. However, it should be noted that these findings may not be directly applicable or reliable for future projects.
Davis et al. (2016) reported a fully feasible assessment of a 5000-acre commercial microalgal biomass production project. The total land area was about 7600 acres accounting for required facilities. The annual production capacity was 38 tons/acre/y with a cultivation productivity of 25 g/m2/d. Davis et al. (2016) concluded the production cost was $121.3 per tonne/hectare for 10-acre pond designs and recommended recycling nutrients to minimize the greenhouse gas footprint. In this context, water, ammonia, and nutrients could be replaced with wastewater as a growth medium for O. pusilla.
Based on the biomass production result of 2.5 g/L in 100% wastewater, the wastewater could substitute for conventional growth media, making the total production cost comparable despite little increased pretreatment costs. Based on observed biomass productivities, the projected annual biomass production could reach 25–30 tonnes/hectare utilizing O. pusilla grown on local municipal wastewater. With a lipid content of 536.8 mg/g DW at 3000 TDS, the projected annual oil yield is estimated at 13.4–16.1 tonnes/hectare. With estimated cultivation cost of $121.3 per tonne/hectare and extraction cost of $500–1000 per tonne, the production cost is projected to be around $0.25–0.5 per kg of oil. At current biodiesel prices of $1.5–3 per kg, this suggests strong potential profitability of the proposed system. Scaled up to a 100-hectare facility, total annual oil production could reach 1340–1610 tonnes, demonstrating potential scalability.
This simple assessment shows the potential for wastewater utilization, as it often exceeds surrounding industry and civilization regions. Excess wastewater spreading causes negative environmental impacts through nutrient leaching, eutrophication, and GHG emissions. Therefore, microalgae cultivation enables nutrient recycling and recovery from wastewater in a cost-effective manner.
Conclusion
Cultivating microalgae in wastewater represents a sustainable and eco-friendly approach for biodiesel production while mitigating wastewater disposal’s environmental consequences. O. pusilla emerges as an auspicious choice for biodiesel production using wastewater as a growth medium, mainly due to its impressive biomass productivity and lipid yield. Moreover, elevating the total dissolved solids (TDS) level in the culture medium can substantially increase lipid yield. The fatty acid composition of the cultivated microalgae aligns with the stipulated values in American and European biodiesel standards, underscoring the feasibility of this method as a cost-effective and environmentally responsible means of generating biofuels. Our findings help fill knowledge gaps regarding growth kinetics, nutrient requirements, and salt tolerance for these specific strains. By elucidating the differences between these two species, our study contributes to the ongoing characterization of microalgae biodiversity for biofuel production. More research is needed to optimize the cultivation conditions and lipid extraction methods to improve the economic feasibility of this approach.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdelsalam I, Elshobary M, Eladawy MM, Nagah M (2019) Utilization of multi-tasking non-edible plants for phytoremediation and bioenergy source-a review. Phyton (B Aires) 88:69–90. https://doi.org/10.32604/phyton.2019.06831
Abomohra AE, Elshobary ME (2019) Microalgae biotechnology for development of biofuel and wastewater treatment. In: Alam MA, Wang Z (eds) In Microalgae Biotechnology for Development of Biofuel and Waste Water Treatment. Springer Singapore, Singapore, pp 293–321. https://doi.org/10.1007/978-981-13-2264-8
Abomohra AE, Abo-Shady AM, Abd El-Moneim AM, Khairy HM, Marey RS (2016) Effect of different culture media on the growth and lipids of the green microalgae, Scenedesmus obliquus and Micractinium reisseri as a feedstock for biodiesel production. Delta J Sci 37:190–198. https://doi.org/10.21608/djs.2016.139939
Abomohra AE, El-Naggar AH, Alaswad SO, Elsayed M, Li M, Li W (2020) Enhancement of biodiesel yield from a halophilic green microalga isolated under extreme hypersaline conditions through stepwise salinity adaptation strategy. Bioresour Technol 310:123462. https://doi.org/10.1016/j.biortech.2020.123462
Abou-Shanab RAII, Ji M-K, Kim H-C, Paeng K-J, Jeon B-H (2013) Microalgal species growing on piggery wastewater as a valuable candidate for nutrient removal and biodiesel production. J Environ Manag 115:257–264. https://doi.org/10.1016/j.jenvman.2012.11.022
Ali EM, Elshobary M, El-Sheekh MM (2023) Algal bioenergy. In: Green Approach to Alternative Fuel for a Sustainable Future. Elsevier, pp 409–432. https://doi.org/10.1016/B978-0-12-824318-3.00021-7
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Ashokkumar V, Salam Z, Tiwari ON, Chinnasamy S, Mohammed S, Ani FN (2015) An integrated approach for biodiesel and bioethanol production from Scenedesmus bijugatus cultivated in a vertical tubular photobioreactor. Energy Convers Manag 101:778–786. https://doi.org/10.1016/j.enconman.2015.06.006
Ashour M, Elshobary ME, El-Shenody R, Kamil AWA, Abomohra AE (2019) Evaluation of a native oleaginous marine microalga Nannochloropsis oceanica for dual use in biodiesel production and aquaculture feed. Biomass Bioenerg 120:439–447. https://doi.org/10.1016/j.biombioe.2018.12.009
Barakat KM, El-Sayed HS, Khairy HM, El-Sheikh MA, Al-Rashed SA, Arif IA, Elshobary ME (2021) Effects of ocean acidification on the growth and biochemical composition of a green alga (Ulva fasciata) and its associated microbiota. Saudi J Biol Sci 28:5106–5114. https://doi.org/10.1016/j.sjbs.2021.05.029
BenMoussa-Dahmen I, Chtourou H, Rezgui F, Sayadi S, Dhouib A (2016) Salinity stress increases lipid, secondary metabolites and enzyme activity in Amphora subtropica and Dunaliella sp. for biodiesel production. Bioresour Technol 218:816–825. https://doi.org/10.1016/j.biortech.2016.07.022
Bigogno C (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60:497–503. https://doi.org/10.1016/S0031-9422(02)00100-0
Chandrasekhar K, Raj T, Ramanaiah SV, Kumar G, Banu JR, Varjani S, Sharma P, Pandey A, Kumar S, Kim S-H (2022) Algae biorefinery: a promising approach to promote microalgae industry and waste utilization. J Biotechnol 345:1–16. https://doi.org/10.1016/j.jbiotec.2021.12.008
Chen H, Wang Q (2021) Regulatory mechanisms of lipid biosynthesis in microalgae. Biol Rev 96:2373–2391. https://doi.org/10.1111/brv.12759
Davis R, Markham J, Kinchin C et al (2016) Process design and economics for the production of algal biomass: algal biomass production in open pond systems and processing through dewatering for downstream conversion. National Renewable Energy Lab. (NREL), Golden. https://doi.org/10.2172/1239893
El Shafay SM, Gaber A, Alsanie WF, Elshobary ME (2021) Influence of nutrient manipulation on growth and biochemical constituent in Anabaena variabilis and Nostoc muscorum to enhance biodiesel production. Sustainability 13:9081. https://doi.org/10.3390/su13169081
El‐Khodary GM, El‐Sayed HS, Khairy HM, El‐Sheikh MA, Qi X, Elshobary ME (2020) Comparative study on growth, survival and pigmentation of Solea aegyptiaca larvae by using four different microalgal species with emphasize on water quality and nutritional value. Aquac Nutr anu.13211. https://doi.org/10.1111/anu.13211
El-Sapagh S, El-Shenody R, Pereira L, Elshobary M (2023) Unveiling the potential of algal extracts as promising antibacterial and antibiofilm agents against multidrug-resistant pseudomonas aeruginosa: in vitro and in silico studies including molecular docking. Plants 12:3324. https://doi.org/10.3390/plants12183324
EL-Seesy AI, Elshobary ME, He Z (2021) Biofuel versus fossil fuel. In: Handbook of algal biofuels Aspects of Cultivation, Conversion, and Biorefinery. Elsevier, pp 181–193. https://doi.org/10.1016/B978-0-12-823764-9.00027-3
El-Sheekh MM, Gheda SF, El-Sayed AE-KB, Abo Shady AM, El-Sheikh ME, Schagerl M (2019) Outdoor cultivation of the green microalga Chlorella vulgaris under stress conditions as a feedstock for biofuel. Environ Sci Pollut Res 26:18520–18532. https://doi.org/10.1007/s11356-019-05108-y
El-Sheekh MM, Galal HR, Mousa ASH, Farghl AAM (2023) Coupling wastewater treatment, biomass, lipids, and biodiesel production of some green microalgae. Environ Sci Pollut Res 30:35492–35504. https://doi.org/10.1007/s11356-023-25628-y
El-Sheekh M, Abomohra AE, El-Azim MA, Abou-Shanab R (2017) Effect of temperature on growth and fatty acids profile of the biodiesel producing microalga Scenedesmus acutus. BASE 233–239. https://doi.org/10.25518/1780-4507.15291
Elshobary ME, Abo-Shady AM, Khairy HM, Essa D, Zabed HM, Qi X, Abomohra AE (2019) Influence of nutrient supplementation and starvation conditions on the biomass and lipid productivities of Micractinium reisseri grown in wastewater for biodiesel production. J Environ Manage 250:109529. https://doi.org/10.1016/j.jenvman.2019.109529
Elshobary ME, El-Shenody RA, Abomohra AE (2020) Sequential biofuel production from seaweeds enhances the energy recovery : a case study for biodiesel and bioethanol production. Int J Energy Res 45:1–11. https://doi.org/10.1002/er.6181
Elshobary ME, Zabed HM, Qi X, El-Shenody RA (2022) Enhancing biomass and lipid productivity of a green microalga Parachlorella kessleri for biodiesel production using rapid mutation of atmospheric and room temperature plasma. Biotechnol Biofuels Bioprod 15:122. https://doi.org/10.1186/s13068-022-02220-z
Enamala MK, Enamala S, Chavali M, Donepudi J, Yadavalli R, Kolapalli B, Aradhyula TV, Velpuri J, Kuppam C (2018) Production of biofuels from microalgae - a review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew Sustain Energy Rev 94:49–68. https://doi.org/10.1016/j.rser.2018.05.012
Essa D, Abo-Shady AM, Khairy HM, Abomohra AE, Elshobary M (2018) Potential cultivation of halophilic oleaginous microalgae on industrial wastewater. Egypt J Bot 58:205–216. https://doi.org/10.21608/ejbo.2018.809.1054
Hawrot-Paw M, Ratomski P, Koniuszy A, Golimowski W, Teleszko M, Grygier A (2021) Fatty acid profile of microalgal oils as a criterion for selection of the best feedstock for biodiesel production. Energies 14:7334. https://doi.org/10.3390/en14217334
Hendy NI (1964) An introductory account of the smaller algae of British Coastal Waters. V. Bacillariophyceae (Diatoms) 1–317
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639. https://doi.org/10.1111/j.1365-313X.2008.03492.x
Huo S, Wang H, Chen J, Hu X, Zan X, Zhang C, Qian J, Zhu F, Ma H, Elshobary M (2022) A preliminary study on polysaccharide extraction, purification, and antioxidant properties of sugar-rich filamentous microalgae Tribonema minus. J Appl Phycol 34:2755–2767. https://doi.org/10.1007/s10811-021-02630-w
Ismail MM, Ismail GA, Elshobary ME (2023) Morpho-anatomical, and chemical characterization of some calcareous Mediterranean red algae species. Bot Stud 64:10. https://doi.org/10.1186/s40529-023-00373-0
Karpagam R, Preeti R, Ashokkumar B, Varalakshmi P (2015) Enhancement of lipid production and fatty acid profiling in Chlamydomonas reinhardtii, CC1010 for biodiesel production. Ecotoxicol Environ Saf 121:253–257. https://doi.org/10.1016/j.ecoenv.2015.03.015
Kessler E, Czygan F-C (1970) Physiological and biochemical contributions to the taxonomy of the genus Chlorella: IV. Utilization of organic nitrogen compounds. Arch Mikrobiol 70:211–216
Khatoon H, Abdu Rahman N, Banerjee S, Harun N, Suleiman SS, Zakaria NH, Lananan F, Abdul Hamid SH, Endut A (2014) Effects of different salinities and pH on the growth and proximate composition of Nannochloropsis sp. and Tetraselmis sp. isolated from South China Sea cultured under control and natural condition. Int Biodeterior Biodegrad 95:11–18. https://doi.org/10.1016/j.ibiod.2014.06.022
Knothe G (2016) Biodiesel and Its Properties. In: Industrial Oil Crops. Elsevier, pp 15–42
Kühl A, Lorenzen H (1964) Handling and culturing of chlorella. In: Methods in Cell Biology. Elsevier, pp 159–187
Markle PJ, Gully JR, Baird RB, Nakada KM, Bottomley JP (2000) Effects of several variables on whole effluent toxicity test performance and interpretation. Environ Toxicol Chem 19:123–132. https://doi.org/10.1002/etc.5620190115
Matos ÂP, Ferreira WB, de Oliveira Torres RC, Morioka LRI, Canella MHM, Rotta J, da Silva T, Moecke EHS, Sant’Anna ES (2015) Optimization of biomass production of Chlorella vulgaris grown in desalination concentrate. J Appl Phycol 27:1473–1483. https://doi.org/10.1007/s10811-014-0451-y
Osman MEH, Abo-Shady AM, Elshobary ME, Abd El-Ghafar MO, Abomohra AE (2020) Screening of seaweeds for sustainable biofuel recovery through sequential biodiesel and bioethanol production. Environ Sci Pollut Res 27:32481–32493. https://doi.org/10.1007/s11356-020-09534-1
Paliwal C, Mitra M, Bhayani K, Bharadwaj SVV, Ghosh T, Dubey S, Mishra S (2017) Abiotic stresses as tools for metabolites in microalgae. Bioresour Technol 244:1216–1226. https://doi.org/10.1016/j.biortech.2017.05.058
Pandit PR, Fulekar MH, Karuna MSL (2017) Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ Sci Pollut Res 24:13437–13451. https://doi.org/10.1007/s11356-017-8875-y
Prescott GW (1987) How to know the freshwater algae, 3rd edn. Wm. C. Brown Co. Publ., Dubuque Iowa
Radwan SS (1978) Coupling of two-dimensional thin-layer chromatography with gas chromatography for the quantitative analysis of lipid classes and their constituent fatty acids. J Chromatogr Sci 16:538–542
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268. https://doi.org/10.1016/j.biortech.2008.06.039
Rodríguez-Palacio MC, Cabrera-Cruz RBE, Rolón-Aguilar JC, Tobías-Jaramillo R, Martínez-Hernández M, Lozano-Ramírez C (2022) The cultivation of five microalgae species and their potential for biodiesel production. Energy Sustain Soc 12:10. https://doi.org/10.1186/s13705-022-00337-5
Senousy HH, Khairy HM, El-Sayed HS, Sallam ER, El-Sheikh MA, Elshobary ME (2023) Interactive adverse effects of low-density polyethylene microplastics on marine microalga Chaetoceros calcitrans. Chemosphere 311:137182. https://doi.org/10.1016/j.chemosphere.2022.137182
Sforza E, Kumkum P, Barbera E, Kumar S (2020) Bioremediation of industrial effluents: how a biochar pretreatment may increase the microalgal growth in tannery wastewater. J Water Process Eng 37:101431. https://doi.org/10.1016/j.jwpe.2020.101431
Sharma AK, Sahoo PK, Singhal S, Joshi G (2016) Exploration of upstream and downstream process for microwave assisted sustainable biodiesel production from microalgae Chlorella vulgaris. Bioresour Technol 216:793–800. https://doi.org/10.1016/j.biortech.2016.06.013
Shu Q, Qin L, Yuan Z, Zhu S, Xu J, Xu Z, Feng P, Wang Z (2018) Comparison of dairy wastewater and synthetic medium for biofuels production by microalgae cultivation. Energy Sources, Part A Recover Util Environ Eff 40:751–758. https://doi.org/10.1080/15567036.2014.907847
Siddiki SYA, Mofijur M, Kumar PS, Ahmed SF, Inayat A, Kusumo F, Badruddin IA, Khan TMY, Nghiem LD, Ong HC, Mahlia TMI (2022) Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: an integrated biorefinery concept. Fuel 307:121782. https://doi.org/10.1016/j.fuel.2021.121782
Smith GM (1950) The fresh-water algae of the United States (2nd ed.). McGraw-Hill Book Company, Inc., New York, p 719. https://doi.org/10.1126/science.113.2938.457
Song M, Pei H, Hu W, Ma G (2013) Evaluation of the potential of 10 microalgal strains for biodiesel production. Bioresour Technol 141:245–251. https://doi.org/10.1016/j.biortech.2013.02.024
Srimongkol P, Sangtanoo P, Songserm P, Watsuntorn W, Karnchanatat A (2022) Microalgae-based wastewater treatment for developing economic and environmental sustainability: current status and future prospects. Front Bioeng Biotechnol 10. https://doi.org/10.3389/fbioe.2022.904046
Srinuanpan S, Cheirsilp B, Prasertsan P, Kato Y, Asano Y (2018) Strategies to increase the potential use of oleaginous microalgae as biodiesel feedstocks: nutrient starvations and cost-effective harvesting process. Renew Energy 122:507–516. https://doi.org/10.1016/j.renene.2018.01.121
Srinuanpan S, Cheirsilp B, Kassim MA (2020) Oleaginous microalgae cultivation for biogas upgrading and phytoremediation of wastewater. In: Microalgae cultivation for biofuels production. Elsevier, pp 69–82. https://doi.org/10.1016/B978-0-12-817536-1.00005-9
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. https://doi.org/10.25607/OBP-1791
Sun Z, Fang X, Li X, Zhou Z (2018) Oleaginous microalgae from dairy farm wastewater for biodiesel production: isolation, characterization and mass cultivation. Appl Biochem Biotechnol 184:524–537. https://doi.org/10.1007/s12010-017-2564-7
Talebi AF, Mohtashami SK, Tabatabaei M, Tohidfar M, Bagheri A, Zeinalabedini M, Hadavand Mirzaei H, Mirzajanzadeh M, Malekzadeh Shafaroudi S, Bakhtiari S (2013) Fatty acids profiling: a selective criterion for screening microalgae strains for biodiesel production. Algal Res 2:258–267. https://doi.org/10.1016/j.algal.2013.04.003
Venkata Mohan S, Devi MP (2014) Salinity stress induced lipid synthesis to harness biodiesel during dual mode cultivation of mixotrophic microalgae. Bioresour Technol 165:288–294. https://doi.org/10.1016/j.biortech.2014.02.103
Venkata Subhash G, Rajvanshi M, Raja Krishna Kumar G, Shankar Sagaram U, Prasad V, Govindachary S, Dasgupta S (2022) Challenges in microalgal biofuel production: a perspective on techno economic feasibility under biorefinery stratagem. Bioresour Technol 343:126155. https://doi.org/10.1016/j.biortech.2021.126155
Wang H, Xiong H, Hui Z, Zeng X (2012) Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresour Technol 104:215–220. https://doi.org/10.1016/j.biortech.2011.11.020
Wang Y, Chen T, Qin S (2013) Differential fatty acid profiles of Chlorella kessleri grown with organic materials. J Chem Technol Biotechnol 88:651–657. https://doi.org/10.1002/jctb.3881
Wang H, Hu X, Cui Y, Sobhi M, Xu X, Zan X, Zhu F, Ni J, Elshobary M, Huo S (2023a) Oil-rich filamentous algae cultivation in anaerobic digestate effluent: inhibition effect of undissociated fatty acids. Algal Res 69:102964. https://doi.org/10.1016/j.algal.2022.102964
Wang H, Hu X, Shao C, Elshobary M, Zhu F, Cui Y, Zhang C, Ni J, Huo S (2023b) Optimizing mixotrophic cultivation of oil-rich Tribonema minus using volatile fatty acids and glycerin: a promising approach for pH-controlling and enhancing lipid productivity. J Clean Prod 402:136733. https://doi.org/10.1016/j.jclepro.2023.136733
Xin C, Addy MM, Zhao J, Cheng Y, Cheng S, Mu D, Liu Y, Ding R, Chen P, Ruan R (2016) Comprehensive techno-economic analysis of wastewater-based algal biofuel production: a case study. Bioresour Technol 211:584–593. https://doi.org/10.1016/j.biortech.2016.03.102
Xu J, Li T, Li C-L, Zhu S-N, Wang Z-M, Zeng EY (2020) Lipid accumulation and eicosapentaenoic acid distribution in response to nitrogen limitation in microalga Eustigmatos vischeri JHsu-01 (Eustigmatophyceae). Algal Res 48:101910
You X, Yang L, Zhou X, Zhang Y (2022) Sustainability and carbon neutrality trends for microalgae-based wastewater treatment: a review. Environ Res 209:112860
Yun C-J, Hwang K-O, Han S-S, Ri H-G (2019) The effect of salinity stress on the biofuel production potential of freshwater microalgae Chlorella vulgaris YH703. Biomass Bioenerg 127:105277. https://doi.org/10.1016/j.biombioe.2019.105277
Zhang L, Cheng J, Pei H, Pan J, Jiang L, Hou Q, Han F (2018) Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renew Energy 115:276–287. https://doi.org/10.1016/j.renene.2017.08.034
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohamed E. H. Osman, Atef M. Abo-Shady, Saly F. Gheda, Samy M. Desoki, and Mostafa E. Elshobary contributed to the study conception and design. Material preparation, data collection and analysis were performed by Samy M. Desoki and Mostafa E. Elshobary. The first draft of the manuscript was written by Samy M. Desoki and Mostafa E. Elshobary. The paper was revised by Mostafa E. Elshobary, Saly F Gheda, Mohamed E. H. Osman and Atef M. Abo-Shady. Mohamed E. H. Osman, Atef M. Abo-Shady, Saly F. Gheda, Samy M. Desoki, and Mostafa E. Elshobary read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohamed E. H. Osman and Mostafa E. Elshobary contributed equally to this work and are co‑first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osman, M.E.H., Abo-Shady, A.M., Gheda, S.F. et al. Unlocking the potential of microalgae cultivated on wastewater combined with salinity stress to improve biodiesel production. Environ Sci Pollut Res 30, 114610–114624 (2023). https://doi.org/10.1007/s11356-023-30370-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30370-6