Abstract
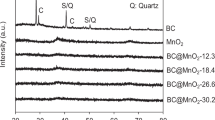
Heavy metal pollution is one of the environmental problems that need to be solved urgently. The adsorption method is thought as the most effective and economical treatment technology. Nature biochar usually showed unsatisfactory adsorption capacity due to its relatively small adsorption capacity and slow adsorption rate. The metal of Mn has been widely applied in the modification of biochar, which could effectively improve the adsorption capacity of biochar. However, leaching of Mn2+ on the adsorbent materials would appear during the adsorption process. And it would increase the risk of secondary pollution. The multifunctional binary modified biochar could improve the adsorption capacity of environmental pollutant removal. In addition, it could also act as a metal support carrier, reducing the risk of secondary pollution. A novel effective biochar loaded by Mg-Mn binary oxide nanoparticles (MgMn2O4@Biochar) was prepared and applied for the Cr(VI) and Pb(II) removal in aqueous solution. The characteristic of MgMn2O4@Biochar was analyzed by SEM, TEM, FTIR, and XRD. The irregular and somewhat flaky shaped particles of different shape and sizes clustered on the surface of MgMn2O4@Biochar appeared. Abundant functional groups of O–H, –C–OH, C–O, and C–OOH could be observed on the surface of MgMn2O4@Biochar. The elements of Mg and Mn elements besides of C, O, and Si elements were presented on the surface of MgMn2O4@Biochar. The wt% of C, O, Mg, Mn, and Si were 42.82%, 48.99%, 2.83%, 4.44%, and 0.93%, respectively. The operational parameters had an important influence on adsorption capacity of Cr(VI) and Pb(II) removal. The results showed that the adsorption capacity of MgMn2O4@Biochar for Cr(VI) and Pb(II) would reach 33.5 mg/g and 536 mg/g, respectively, within 360 min. Additionally, the adsorption processes of Cr(VI) and Pb(II) in solution could be described with pseudo-second-order. For Cr(VI), the Langmuir model was suitable to the adsorption process. However, the adsorption process of Pb(II) in solution could be described with Freundlich model. Furthermore, it could be concluded that the possible mechanism of Cr(VI) and Pb(II) removal by MgMn2O4@Biochar was physical adsorption, surface complexation reaction, and electrostatic adsorption.






Similar content being viewed by others
Data availability
The data and materials presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
References
Aboulkas A, Hammani H, El Achaby M, Bilal E, Barakat A, El Harfi K (2017) Valorization of algal waste via pyrolysis in a fixed-bed reactor: production and characterization of bio-oil and bio-char. Bioresour Technol 243:400–408
Charbonnet JA, Duan Y, Sedlak DL (2020) The use of manganese oxide-coated sand for the removal of trace metal ions from stormwater. Environ Sci Wat Res 6:593–603
Chen H, Gao Y, Li J, Fang Z, Bolan N, Bhatnagar A, Gao B, Hou D, Wang S, Song H, Yang X, Shaheen SM, Meng J, Chen W, Rinklebe J, Wang H (2022a) Engineered biochar for environmental decontamination in aquatic and soil systems: a review. Carbon Res 1:4
Chen Z, He X, Li Q, Yang H, Liu Y, Wu L, Liu Z, Hu B, Wang X (2022b) Low-temperature plasma induced phosphate groups onto coffee residue-derived porous carbon for efficient U(VI) extraction. J Environ Sci 122:1–13
Chen YQ, Yang HP, Wang XH, Chen W, Chen HP (2016) Biomass pyrolytic polygeneration system: adaptability for different feedstocks. Sustain Energ Fuels 30:414–422
Duan W, Chen G, Chen C, Sanghvi R, Iddya A, Walker S, R=Liu H., Ronen A., Jassby D. (2017) Electrochemical removal of hexavalent chromium using electrically conducting carbon nanotube/polymer composite ultrafiltration membranes. J Membr Sci 531:160–171
Dutta D, Kundu D, Jana BB, Lahiri S, Bhakta JN (2022) Greenhouse-temperature induced manure driven low carbon footprint in aquaculture mesocosm. Carbon Res 1:18
Fakhri A (2015) Investigation of mercury (II) adsorption from aqueous solution onto copper oxide nanoparticles: optimization using response surface methodology. Process Saf Environ Prot 93:1–8
Ge T, Jiang Z, Shen L, Li J, Lu Z, Zhang Y, Wang F (2021) Synthesis and application of Fe3O4/FeWO4 composite as an efficient and magnetically recoverable visible light-driven photocatalyst for the reduction of Cr(VI). Sep Purif Technol 263:118401
Ge T, Shen L, Li J, Zhang Y, Zhang Y (2022) Morphology-controlled hydrothermal synthesis and photocatalytic Cr(VI) reduction properties of α-Fe2O3. Colloid Surf A 635:128069
Han TU, Kim J, Kim K (2020) Freezing-accelerated removal of chromate by biochar synthesized from waste rice husk. Sep Purif Technol 250:117233
Hu B, Wang H, Liu R, Qiu M (2021) Highly efficient U(VI) capture by amidoxime/carbon nitride composites: evidence of EXAFS and modeling. Chemosphere 274:129743
Huang D, Li B, Wu M, Kuga S, Huang Y (2018) Graphene oxide-based Fe-Mg (Hydr) oxide nanocomposite as heavy metals adsorbent. J Chem Eng Data 63:2097–2105
Jin X, Liu R, Wang H, Han L, Qiu M, Hu B (2022) Functionalized porous nanoscale Fe3O4 particles supported biochar from peanut shell for Pb(II) ions removal from landscape wastewater. Environ Sci Pollut R 29:37159–37169
Jin G, Gu P, Qin L, Li K, Guan Y, Su H (2023) Preparation of manganese-oxides-coated magnetic microcrystalline cellulose via KMnO4 modification: improving the counts of the acid groups and adsorption efficiency for Pb(II). Int J Biol Macromol 239:124277
Khan NA, Khan SU, Ahmed S, Farooqi IH, Dhingra A, Hussain A, Changani F (2019) Applications of nanotechnology in water and wastewater treatment: a review. Asian J Water Environ Pollut 16:81–86
Khan SU, Farooqi IH, Ayub S (2017) Studies on application of Fe based binary oxide nanoparticles for treatment of lead (Pb2+) contaminated water—a batch study. Mater Today Proc 4:9650–9655
Khan SU, Khalid M, Zaidi R, Farooqi IH, Azam A, Ayub S (2021) Applicability of Mn-Mg binary oxide nanoparticles for the adsorptive removal of copper and zinc from aqueous solution. Mater Today Proc 47:1500–1506
Khan SU, Zaidi R, Hassan SZ, Farooqi IH, Azam A (2016) Application of Fe-Cu binary oxide nanoparticles for the removal of hexavalent chromium from aqueous solution. Water Sci Technol 74:165–175
Kuang J, Ba Z, Li Z, Jia Y, Wang Z (2019) Fabrication of a superhydrophobic Mg-Mn layered double hydroxides coating on pure magnesium and its corrosion resistance. Surf Coat Technol 361:75–82
Kurniawan TA, Sillanpää MET, Sillanpää M (2012) Nanoadsorbents for remediation of aquatic environment: local and practical solutions for global water pollution problems. Crit Rev Environ Sci Technol 42:1233–1295
Kwon OH, Kim JO, Cho DW, Kumar R, Baek SH, Kurade MB, Jeon BH (2016) Adsorption of As(III), As(V) and Cu(II) on zirconium oxide immobilized alginate beads in aqueous phase. Chemosphere 160:126–133
Li X, Jia Y, Zhou M, Su X, Sun J (2020) High-efffciency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation. J Hazard Mater 397:122764
Li M, Kuang S, Dong J, Ma H, Kang Y (2023) Performance and mechanisms of Cr(VI) removal by nano-MnO2 with different lattices. J Mol Struct 1275:134624
Liu FL, Hua S, Wang C, Qiu MQ, Jin LM, Hu BW (2021a) Adsorption and reduction of Cr(VI) from aqueous solution using cost-effective caffeic acid functionalized corn starch. Chemosphere 279:130539
Liu J, Liu H, Yang X, Jia X, Cai M, Bao Y (2021b) Preparation of Si-Mn/biochar composite and discussions about characterizations, advances in application and adsorption mechanisms. Chemopshere 281:130946
Liu G, Dai Z, Liu X, Dahlgren RA, Xu J (2022a) Modification of agricultural wastes to improve sorption capacities for pollutant removal from water—a review. Carbon Res 1:24
Liu K, Ran Q, Li F, Shaheen SM, Wang H, Rinklebe J, Liu C, Fang L (2022b) Carbon-based strategy enables sustainable remediation of paddy soils in harmony with carbon neutrality. Carbon Res 1:12
Liu RR, Zhang YH, Hu BW, Wang H (2022c) Improved Pb(II) removal in aqueous solution by sulfide@biochar and polysaccharose-FeS@ biochar composites: efficiencies and mechanisms. Chemosphere 287:132087
Liu WJ, Jiang H, Yu HQ (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285
Liu Z, Xu Z, Xu L, Buyong F, Chay TC, Li Z, Cai Y, Hu B, Zhu Y, Wang X (2022d) Modified biochar: synthesis and mechanism for removal of environmental heavy metals. Carbon Res 1:8
Lu X, Liu X, Zhang W, Wang X, Wang S, Xia T (2019) The residue from the acidic concentrated lithium bromide treated crop residue as biochar to remove Cr (VI). Bioresour Technol 296:122348
Mahmoud ME, Abou Ali SAA, Elweshahy SMT (2018) Microwave functionalization of titanium oxide nanoparticles with chitosan nanolayer for instantaneous microwave sorption of Cu(II) and Cd(II) from water. Int J Biol Macromol 111:393–399
Maneechakr P, Mongkollertlop S (2020) Investigation on adsorption behaviors of heavy metal ions (Cd2+, Cr3+, Hg2+ and Pb2+) through low-cost/active manganese dioxide-modiffed magnetic biochar derived from palm kernel cake residue. J Environ Chem Eng 8:104467
Qian L, Liu S, Zhang W, Chen Y, Ouyang D, Han L, Yan J, Chen M (2019) Enhanced reduction and adsorption of hexavalent chromium by palladium and silicon rich biochar supported nanoscale zero-valent iron. J Colloid Interface Sci 533:428–436
Qiu M, Hu B, Chen Z, Yang H, Wang X (2021) Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar 3:117–123
Qiu M, Liu L, Ling Q, Cai Y, Yu S, Wang S, Fu D, Hu B, Wang X (2022) Biochar for the removal of contaminants from soil and water: a review. Biochar 4:19
Selvi K, Pattabhi S, Kadirvelu K (2001) Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour Technol 80:87–89
Shahsavar A, Mohajeri A (2018) Impact of position and number of nitrogen atom substitution on the curvature and hydrogen adsorption properties of metallized borophene. J Mater Sci 6:4540–4553
Shao J, Zhang J, Zhang X, Feng Y, Zhang H, Zhang S, Chen H (2018) Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation. Fuels 224:138–146
Shi Y, Zhang T, Ren H, Kruse A, Cui R (2017) Polyethylene imine modified hydrochar adsorption for chromium (VI) and nickel (II) removal from aqueous solution. Bioresour Technol 247:370–379
Valentín-Reyes J, García-Reyes RB, García-Gonzãlez A, Soto-Regalado E, CerinoCórdova F (2019) Adsorption mechanisms of hexavalent chromium from aqueous solutions on modiffed activated carbons. J Environ Manag 236:815–822
Vellaichamy S, Palanivelu K (2011) Preconcentration and separation of copper, nickel and zinc in aqueous samples by flame atomic absorption spectrometry after column solid-phase extraction onto MWCNTs impregnated with D2EHPA-TOPO mixture. J Hazard Mater 185:1131–1139
Wang B, Li F, Wang L (2019) Enhanced hexavalent chromium (Cr(VI)) removal from aqueous solution by Fe-Mn oxide-modiffed cattail biochar: adsorption characteristics and mechanism. Chem Ecol 36:1699537
Wang H, Gao B, Wang S, Fang J, Xue Y, Yang K (2015) Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour Technol 197:356–362
Wang X, Liang D, Wang Y, Peijnenburg WJGM, Monikh FA, Zhao X, Dong Z, Fan W (2022) A critical review on the biological impact of natural organic matter on nanomaterials in the aquatic environment. Carbon Res 1:13
Wang Y, Su Y, Fang W, Zhang Y, Li X, Zhang G, Sun W (2020) SnO2/SnS2 nanocomposite anchored on nitrogen-doped RGO for improved photocatalytic reduction of aqueous Cr(VI). Powder Technol 363:337–348
Wu F, Li F, Zhao X, Bolan NS, Fu P, Lam SS, Mašek O, Ong HC, Pan B, Qiu X, Rinklebe J, Tsang DCW, Zwieten LV, Vithanage M, Wang S, Xing B, Zhang G, Wang H (2022) Meet the challenges in the “Carbon Age”. Carbon Res 1:1
Xiang W, Zhang X, Chen K, Fang J, Gao B (2019) Enhanced adsorption performance and governing mechanisms of ball-milled biochar for the removal of volatile organic compounds (VOCs). Chem Eng J 385:123842
Xiang W, Zhang X, Chen J, Zou W, He F, Hu X, Tsang DCW, Ok YS, Gao B (2020) Biochar technology in wastewater treatment: a critical review. Chemosphere 252:126539
Xu Z, Xu X, Tsang DCW, Yang F, Zhao L, Qiu H, Cao X (2020) Participation of soil active components in the reduction of Cr(VI) by biochar: differing effects of iron mineral alone and its combination with organic acid. J Hazard Mater 384:121455
Yan LL, Kong L, Qu Z, Li L, Shen GQ (2015) Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sustain Chem Eng 3:125–132
Yang P, Guo DB, Chen ZH, Cui BH, Xiao B, Liu SM, Hu M (2017) Removal of Cr (VI) from aqueous solution using magnetic biochar synthesized by a single step method. J Dispers Sci Technol 38:1665–1674
Yao L, Zhang YC, Li J, Chen Y (2014) Photocatalytic properties of SnS2/SnO2 nanocomposite prepared by thermal oxidation of SnS2 nanoparticles in air. Sep Purif Technol 122:1–5
Yin Q, Liu M, Li Y, Li H, Wen Z (2021) Computational study of phosphate adsorption on Mg/Ca modified biochar structure in aqueous solution. Chemosphere 269:129374
Zhang F, Zhang Y, Wang Y, Zhu A, Zhang Y (2022a) Efficient photocatalytic reduction of aqueous Cr (VI) by Zr4+ doped and polyaniline coupled SnS2 nanoflakes. Sep Purif Technol 283:120161
Zhang L, Li W, Cao H, Hu D, Chen X, Guan Y, Tang J, Gao H (2019) Ultraefficient sorption of Cu(2+) and Pb(2+) ions by light biochar derived from Medulla tetrapanacis. Bioresour Technol 291:121818
Zhang M, Gao B, Yao Y, Inyang M (2013) Phosphate removal ability of biochar/MgAlLDH ultra-fine composites prepared by liquid-phase deposition. Chemosphere 92:1042–1047
Zhang T, Chen J, Xiong H, Yuan Z, Zhu Y, Hu B (2021a) Constructing new Fe3O4@MnOx with 3D hollow structure for efficient recovery of uranium from simulated seawater. Chemosphere 283:131241
Zhang Y, Meng Y, Ma L, Ji H, Lu X, Pang Z, Dong C (2021b) Production of biochar from lignocellulosic biomass with acidic deep eutectic solvent and its application as efficient adsorbent for Cr(VI). J Clean Prod 324:129270
Zhang X, Lv L, Qin Y, Xu M, Jia X, Chen Z (2018) Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour Technol 256:1–10
Zhang X, Yang X, Yuan X, Tian S, Wang X, Zhang H, Han L (2022b) Effect of pyrolysis temperature on composition, carbon fraction and abiotic stability of straw biochars: correlation and quantitative analysis. Carbon Res 1:17
Zhong M, Li M, Tan B, Gao B, Qiu Y, Wei X, Hao H, Xia Z, Zhang Q (2021) Investigations of Cr(VI) removal by millet bran biochar modified with inorganic compounds: momentous role of additional lactate. Sci Total Environ 793:148098
Zou S, Wu Y, Yang M, Li C, Tong J (2009) Thermochemical catalytic liquefaction of the marine microalgae dunaliella tertiolecta and characterization of bio-oils. Energy Fuel 23:3753–3758
Funding
This work is supported by the Natural Science Foundation of Zhejiang Province, China (LGF20C030001).
Author information
Authors and Affiliations
Contributions
Muqing Qiu is responsible for ensuring that the descriptions are accurate and agreed by all authors. Weijuan Guo, Ling Yan, and Yujun Chen write original draft. Yiyang Shen, Xinyu Ren, and Yefeng Zhou are performing the experiments and data collection. Baowei Hu revises paper. The first two authors of Weijuan Guo and Ling Yan equally contributed to this work.
Corresponding author
Ethics declarations
Ethical approval
This section is “not applicable” for this study.
Consent to participate
Not applicable.
Consent for publication
All authors reviewed and approved the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Ioannis A. Katsoyiannis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, ., Yan, L., Chen, Y. et al. Effective elimination of hexavalent chromium and lead from solution by the modified biochar with MgMn2O4 nanoparticles: adsorption performance and mechanism. Environ Sci Pollut Res 30, 96350–96359 (2023). https://doi.org/10.1007/s11356-023-29264-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29264-4