Abstract
An exponential rise in global pollution and industrialization has led to significant economic and environmental problems due to the insufficient application of green technology for the chemical industry and energy production. Nowadays, the scientific and environmental/industrial communities push to apply new sustainable ways and/or materials for energy/environmental applications through the so-called circular (bio)economy. One of today’s hottest topics is primarily valorizing available lignocellulosic biomass wastes into valuable materials for energy or environmentally related applications. This review aims to discuss, from both the chemistry and mechanistic points of view, the recent finding reported on the valorization of biomass wastes into valuable carbon materials. The sorption mechanisms using carbon materials prepared from biomass wastes by emphasizing the relationship between the synthesis route or/and surface modification and the retention performance were discussed towards the removal of organic and heavy metal pollutants from water or air (NOx, CO2, VOCs, SO2, and Hg0). Photocatalytic nanoparticle–coated biomass-based carbon materials have proved to be successful composites for water remediation. The review discusses and simplifies the most raised interfacial, photonic, and physical mechanisms that might take place on the surface of these composites under light irradiation. Finally, the review examines the economic benefits and circular bioeconomy and the challenges of transferring this technology to more comprehensive applications.
Similar content being viewed by others
Introduction
Production process through the linear economy for many decades has led to serious environmental, economic, and ecosystem perturbations (Jørgensen et al. 2018; Turner 2018). Linear economy generates huge quantities of waste in the aquatic and atmospheric environment. Hence, the scientific and industrial communities worldwide actively search for green approaches to decrease the quantity and hazardous of generated waste and their possible recycling (Nabgan et al. 2023). The concept of “zero waste theory” is based on using green and recyclable products and valorizing natural and available resources (Romano et al. 2019). As a result, the bioeconomy European Union must include sustainability and circularity at the core of its industrial makeup to effectively transition from a linear to a circular economy. By 2060, international energy agencies anticipated that the world’s need for bioenergy would multiplied (Brahma et al. 2022), suggesting a need to switch to renewable feedstock for heat generation, chemicals, fuels, and electricity, such as lignocellulosic biomass, is gaining more and more attention (Djellabi et al. 2022). Lignocellulosic biomass is a carbon-rich material that can be employed in the sustainable route for bioenergy production and high-value products as well as multifunctional materials for environmental remediation (Bhatnagar et al. 2015; Nouacer et al. 2015; Khelaifia et al. 2016; Wang et al. 2021).
The production of carbon materials from lignocellulosic biomass wastes has gained attention in recent years, particularly due to its low cost and relative earth abundance (Ziati et al. 2012; Yahya et al. 2015; Ikram et al. 2022). Lignocellulosic biomass mainly contains three components: lignin, cellulose, and hemicellulose, wherein lignin is the central part. Lignin is rich in carbon atoms compared to the other components, and therefore, it is regarded as the best precursor to produce activated carbon (Ikram et al. 2022). The synthesis of high-quality activated carbon significantly depends on different factors, such as the starting carbonaceous precursor, carbonization and activation approaches, and other synthesis conditions (Carrott and Carrott 2007). In fact, an extensive list of biomass wastes was converted into activated carbon, such as date stones (Abderrahim et al. 2022a), olive pits (Redondo et al. 2015), coconut shells (Gratuito et al. 2008), lignin (Carrott and Carrott 2007), barley straw (Pallarés et al. 2018), orange peels (Köseoğlu and Akmil-Başar 2015), soybean oil cake (Tay et al. 2009), tomato (Sayğılı and Güzel 2016), chestnut shell (Duan et al. 2021), wood (Danish and Ahmad 2018), palm shell (Hamada et al. 2020), and walnut shell (Plaza et al. 2010). Different approaches are used for the activation to ensure effective carbonization, including the chemical, physical, and physicochemical ones. In terms of chemical activation, several agents can be used, such as KOH (Khalil et al. 2013), FeCl3 (Bedia et al. 2020), ZnCl2 (Caturla et al. 1991), H3PO4 (Yorgun and Yıldız 2015), NaOH (Islam et al. 2017), K2CO3 (Sayğılı and Sayğılı 2019), and K2B4O7 (Gao et al. 2015). In addition, the synthesis method significantly affects several features, such as the surface area, internal porosity, oxygenated functional groups, and physical properties (González-García 2018). Different approaches were reported to assist the activation and carbonization, such as hydrothermal (Hoekman et al. 2011), microwave (Paramasivan 2022), ultrasonic (Xu et al. 2019), and vacuum (Yousaf et al. 2021).
Lignocellulosic biomass has been extensively used as support for photocatalytic nanoparticles to improve the pollutant removal efficiency during environmental remediation. TiO2/carbon composites are some of the successful photocatalytic materials for water purification because of the synergistic effects in terms of enhanced light absorption, excellent adsorptive ability, and promoted charges transferred (Puma et al. 2008; Lisowski et al. 2018; Djellabi et al. 2019a, b, c, d; Djellabi et al. 2019a, b, c, d; Djellabi et al. 2021a, b, c). Several photocatalytic mechanistic pathways have been reported regarding oxidation and reduction of pollutants on the surface of titania-carbon materials (Matos et al. 2010; Asiltürk and Şener 2012; Djellabi et al. 2019a, b, c, d). Besides, a wide list of photocatalysts has been supported on the surface of lignocellulosic biomass or lignocellulosic biomass–based activated carbon such as ZnO (Raizada et al. 2014), Ag3PO4 (Abderrahim et al. 2022b), CuO (Khaled et al. 2020), g-C3N4 (Pi et al. 2015), and MoS2 (Ye et al. 2019).
The present review discusses the valorization of biomass from both fundamental and applied research. It includes most of recent data on the synthesis of carbon based materials to be used for environmental application. Differently to previous reported reviews, we stressed in this review the mechanistic pathways that take place during the synthesis of carbon materials from biomass wastes. In addition, it provides the most a summary regarding the mechanistic pathways taking place for the removal of materials through simple adsorption or photocatalytic route from air and water. The adsorption mechanisms towards the removal of organic pollutants, heavy metals, CO2, VOCs, NOx, and mercury are systematically reviewed. In-depth discussion was held on the photocatalytic behavior of semiconductor-supported lignocellulosic biomass. The most important uses of valorized lignocellulosic biomass–based materials for adsorption and photocatalytic removal of pollutants from the aquatic and atmospheric environment are outlined in the paper.
Synthesis of carbon from lignocellulose biomass
Due to the urgent demand for materials for environmental remediation and the high cost of available commercial materials, a lot of studies have been reported by the scientific community to find sustainable and low-cost alternative routes to fabricate highly adsorptive or functionalized carbons. The valorization of lignocellulosic biomass–based agricultural or industrial wastes has been a hot topic over the last few years. Lignocellulosic biomass contains cellulose, hemicellulose, and lignin. After cellulose, lignin is considered an abundant carbon source material, annually producing 40–50 million tons (Carrott and Carrott 2007). The use of lignocellulosic biomass as a precursor for producing carbon materials is a successful option because of the rich carbon content in lignin. Numerous sources of lignocellulosic biomass can be used to produce activated carbon with a high adsorption capacity, such as durian shells (Chandra et al. 2007), coconut shells (Li et al. 2008), and food waste (Kosheleva et al. 2019). Palm kernel shell (Sumathi et al. 2009), rice husk (Dahlan et al. 2006; Heo and Park 2015), pistachio-nut shell (Yang and Lua 2003), olive pits (Djellabi et al. 2019a, b, c, d), date stones (Khelaifia et al. 2016), palm tree (Nouacer et al. 2015), and wood (Danish and Ahmad 2018, Musa et al. 2019). Activated carbon derived from lignocellulosic biomass is used in a broad range of applications for both industrial and residential uses, including wastewater treatment (Thompson et al. 2016; Wong et al. 2018), air pollution treatment(Devinny et al. 2017), petroleum refining (Cagnon et al. 2009), storage (Zieliński et al. 2005), and catalysis (Tsai et al. 1998). The advantages of activated carbon materials are their stability, high surface area up to 3000 m2/g, and porosity (El Hadrami et al. 2022). In general, biomass conversion into carbon materials passes through two steps, decomposition and evaporation followed by carbon condensation (Fig. 1). In this section, the most common synthesis approaches of chars and activated carbons from biomass were summarized and discussed.
Carbonization
Carbonization, also known as pyrolysis, is a process used to raise the carbon yield of a carbonaceous material by eliminating non-carbon elements under high temperatures resulting in an observable increase in the initial porosity obtained char before the subsequent improvement during the activation phase. Since the carbonization process has a significant impact on the final product, it is crucial to select the parameters carefully. The most significant effects on carbonization are the temperature range and heating rate, with residence time and inert gas flow rate also having a significant impact (Wang et al. 2020a, b, c, d, e, f).
To avoid biomass oxidation and to minimize the emission of volatile gases and water during the carbonization process, an oxygen-free environment must be maintained as much as is practicable. As shown in Fig. 2a, pyrolysis involves four stages: (i) dehydrating moisture from biomass at temperatures below 200 °C; (ii) decomposition of biomass structure to release volatiles and organic acids at temperatures between 170 and 270 °C; (iii) advanced decomposition at 270–350 °C to release liquids and gas, ending up with biochar generation; and (iv) removal of organic components to form activated carbon at temperature up to 700 °C (Ikram et al. 2022). It is challenging to create a perfectly inert environment for continuous pyrolysis since oxygen is present in the vacuum area of bulk biomass (Lua et al. 2006). Therefore, to evaluate product yields and quality, it is crucial to understand how oxygen impacts biomass pyrolysis, as a study has reported that the presence of oxygen during pyrolysis contributes to an increase in both the specific heat capacity and total pore volume (Zhang et al. 2016a, b). Also, the treatment of lignocellulosic biomass at high-temperature values can destroy many oxygen functional groups that in turn change the surface chemistry of the produced carbon. Figure 2 b shows that the decomposition of oxygen functional groups occurs at different calcination temperature values.

Reproduced with permission from Shafeeyan et al. (2010); copyright 2023 Elsevier
a Main steps of biomass pyrolysis to produce activated carbon. b Decomposition of oxygen functional groups on the surface of biomass materials as a function of heat treatment.
Based on the heating rate, pyrolysis can be divided into classes: slow, fast, flash, and intermediate (Laird et al. 2009). At higher temperatures, the yield of biochar is reduced as it has a higher carbon content as a consequence of the deeper devolatilization of the biomass precursor (Colantoni et al. 2016). In general, to control the biochar yield, low heating rates (from 10 to 15 °C/min) are applied along with a medium carbonization temperature (Ioannidou and Zabaniotou 2007). The characteristics of biochar, such as the surface chemistry, pore volume, pore size distribution, and elemental composition, are all mainly influenced by the pyrolysis temperature (Ahmad et al. 2013; Shen et al. 2017).
Torrefaction
Torrefaction is a mild pyrolysis technique used to convert the biomass’s amorphous and crystalline regions to hydrophobic products with high energy density (Rousset et al. 2012). The principal advantages of this biomass pre-treatment are as follows: (i) reduced transportation costs, (ii) low grinding energy, (iii) low milling cost, (iv) cheap chemical treatment and low waste generation, and (v) rapid reactivity, besides to an east to scale and non-corrosive process (Nunes et al. 2014; Acharya et al. 2015). Torrefied by-product yields and attributes depend on several factors, including the biomass type, particle size, processing temperature and time, heating rate, and working atmosphere composition. Much research has been done to determine how these operational factors affect the torrefaction (Shankar Tumuluru et al. 2011; Olugbade and Ojo 2020). It was observed that reducing the temperature at which raw materials are prepared increases the yields and enables the production of a more energetically dense material (Krysanova et al. 2019). The carbon content of the materials increases with the torrefaction time, increasing also the calorific value. Chen and Kuo (2010) concluded that the primary process of biomass decomposition takes place in the first hour and then begin to slow down. The size of the biomass particles is another critical factor in biomass torrefaction that influences the mechanism of process. Wang et al. reported that a greater particle size affected the reactor’s efficiency and decreased the energy yield of biochars. The biomass’s structure and composition have an impact on the torrefaction process as well. It was discovered that alkali metals, like potassium and sodium, present in the biomass accelerated its decomposition during the torrefaction (Wang et al. 2017). Particularly, De Macedo et al. claimed that potassium influences how biopolymers interact, reducing the torrefaction period and increasing the output of important chemicals like guaiacol (De Macedo et al. 2018). The structural content of the biomass determines the temperature range of torrefaction. It has been demonstrated that biomass with a high content of hemicelluloses is effectively treated with moderate torrefaction (between 200 and 235 °C). In contrast, materials with a high cellulose and lignin content benefit most from heavy torrefaction (between 235 and 275 °C) (Chen and Kuo 2010). Currently, two types of torrefaction are employed: dry and wet.
Dry torrefaction
In nature, the process of dry torrefaction is one of the thermochemical conversion processes. This process occurs in the presence of an inert gas at temperatures ranging from 200 to 300 °C for around 30 to 60 min. In this sense, only 10% biomass energy is lost as gases (Bergman et al. 2005). Consequently, the torrefied solid product’s specific energy density increases when the biomass is heated at about 50 °C per min. Dry torrefaction of biomass causes several reactions, such as decarboxylation, dehydration, decarbonylation, demethoxylation, intermolecular rearrangement, condensation, and aromatization chemicals (Chen and Kuo 2010; Acharya et al. 2015). Typically, torrefaction is the initial pyrolysis process; hence, the end product, which still contains some volatile organic components, is not referred to as “biochar.” Generally speaking, the physicochemical properties of torrefied biomass fall between those of raw biomass and those of biochar (Kambo and Dutta 2015).
Wet torrefaction
Wet torrefaction (WT) is known as hydrothermal torrefaction (HTT) or hydrothermal carbonization (HTC) is carried out in the presence of an inert gas and subcritical pressured water vessel under 2 to 10 MPa at temperatures between 180 and 265 °C for treatment times ranging from five minutes to several hours. (Mumme et al. 2011; Xiao et al. 2012). According to Akshay et al. subcritical water has good solubility property in liquid form because of its dielectric point and high density compared to its vapor form (Jain et al. 2016). Because of its higher ionization constant, water can hydrolyze organic molecules at high temperatures, and acids or bases can speed up this process. Due to the creation of different organic acids, including acetic, levulinic, and formic, during the hydrothermal carbonization process, a pH reduction is frequently observed. These organic acids help to accelerate the hydrolysis and fragmentation of oligomers and monomers into smaller pieces that have little effect on lignin. As a result, solid biochar is produced with lower equilibrium moisture content than raw biomass (Demir-Cakan et al. 2009). Table 1 summarizes the main conditions and factors in dry and wet torrefaction approaches.
Microwave-assisted carbonization
Microwave heating systems have recently gained acceptance among scientists as an alternative to conventional heating sources and reactors attributed mainly to various inherent advantages, such as quick heating, selective heating, high controllability, low thermal inertia, and good treatment effects (Hejazifar et al. 2011). The microwave heat production is achieved at low-energy electromagnetic waves, termed microwaves have wavelengths between 0.001 and 0.3 m and frequencies between 1000 and 300,000 MHz (Komarov 2012). Instruments for measuring microwaves in laboratories (and homes) almost exclusively use microwaves with the frequency of 2450 MHz (or 12.2 cm wavelength) (Li et al. 2016a, b). Like all the electromagnetic waves, microwaves move at the speed of light (300,000 km/s) and consist of two oscillating field’s perpendicular to one another: an electric field and a magnetic field. The only effect of microwave photons, which have a low energy of 0.03–0.00003 kcal/mol, is the kinetic molecular excitation.
With the help of these two fields, microwaves can produce heat. The electric field interacts with molecules in dipolar rotation and ionic conduction. In dipolar rotation, each rotating molecule pushes with the others to line up its dipole with the oscillating electric field. This friction generates heat (Li et al. 2016a, b). Energy transmission is more effective with microwaves than conventional heating methods because they directly interact with the components of a reaction mixture (Fig. 3) (Espinosa-Iglesias et al. 2015). Thermal conductivity underlies the conventional heating methods, involving the transfer of heat first from source to the vessel and then from vessel to the solution (Yang et al. 2010). The generated volatile matter moves from the particle’s interior at a high temperature to its surroundings with a lower temperature (An et al. 2020a, b). Thus, the thermal gradient problem with conventional heating techniques is resolved, improving the char yield. Biochar yield in microwave carbonization is primarily affected by the heating rate and radiation power (Ouyang et al. 2020). Table 2 summarizes the main advantages and disadvantages of the conventional and microwave-assisted carbonization.
Mechanism of conductive heating and microwave heating (Omoriyekomwan et al. 2021); copyright 2023 Elsevier
Activation
The purpose of the activation of biochar is to enhance the porosity and the surface area of the carbon materials, as well as the functionalization of the surface (Ahmed 2016). The activation procedure consists of these three basic steps: Firstly, since the elementary carbon crystal surface interact with the activation agents, viscous substances that clog pores must be removed, which is followed be the heating of the elementary carbon crystal and lastly the oxidation of the carbon species (Yang et al. 2010). Temperature and holding time significantly depends on the type of precursor, binding, activation agent, and target adsorbate used, playing a significant role in the activation stage (Kalderis et al. 2008). The most important thing is to determine the correct temperature and time, producing the significant specific surface areas, adequate pore formation, and surface functional groups without reducing the mechanical stability of the AC. The activation can be done through physical, chemical, or physiochemical routes. Table 3 summarizes selected studies on the activation of biomass through these synthetic routes.
Physical activation
Physical activation is a two-step process, beginning with carbonization, followed by an activation at high temperatures in the presence of an oxidation agents such as steam, CO2, air, or a combination of them (Yin et al. 2014; Balahmar et al. 2017). Physical activation is primarily affected by the type of carbon precursor, particle size, gas flow, heating rate, activation time, and temperature and activating agent. It impacts on the produced carbons’ surface properties and porosity. According to Aworn et al. high-density materials, like shells and seeds, are the best activated using steam, whereas fibrous and low-density materials, like straws and husks, are better suited for CO2 activation. However, physical activation has disadvantages, including a high activation temperature, a prolonged processing time, a limited carbon yield, and a limited specific surface area (Aworn et al. 2008).
Chemical activation
In chemical activation techniques, biomass is impregnated with activating chemical agents. These reagents combine decomposition, dehydration, and complexation with organic carbon molecules of precursor materials. Subsequently, the chemically treated biomass is heated in an inert environment to produce activated carbons with a well-developed porous structure and a large surface area (Danish and Ahmad 2018). Moreover, the introduction of pores or surface chemical groups depends on the activating chemicals during activation. Alkaline, acidic, neutral, and self-activating chemical regulators have all been employed. Alkaline activating agents are divided into three types, namely (i) strong activators, including KOH and NaOH; (ii) mid activators, such as K2CO3 and Na2CO3; and (iii) weak alkaline activating agents, such as K2SiO3, Na2SiO3, K2B4O7, C4H6K2O7, CH3COOK, and C6H5K3O7 (Arslanoğlu 2019). According to various previous studies, KOH has been the most useful alkaline agent to create super-activated carbons (Gao et al. 2020; Jawad et al. 2021). Most of this agent will be transformed into K2CO3 and K2O during the activation process: carbon atoms reduce these two byproducts to form the metallic vapor potassium, responsible for pore developments (Arslanoğlu 2019).
-
Acidic activating agents include H3PO4, H4P2O7, HPO3, H6P4O13, C6H18O24P6, and H2SO4. One of the successful acidic activating agents is H3PO4 because of its positive effects on textural and surface chemistry. At heating treatment above 217 °C, polyphosphoric acid is formed by condensing two or more H3PO4 molecules with the elimination of H2O. This formed agent exhibits strong corrosivity, which boosts volatile components’ oxidation and carbonization, creating more pores or transforming micropores into mesopores (Liu et al. 2012; Gao et al. 2020).
-
Neutral activating agents include FeCl3, CaCl2, MgCl2, NH4Cl, K2SO4, KMnO4, KHC4H4O6 NaNH2, NaH2PO4 and KH2PO4, etc. Yuan et al. reported that the development of pores using neutral activating agents is directly related to the reaction between the positive ions (e.g., Fe3+, Mn7+, Mg2+, Na+) at their oxidation state with carbon atoms via reduction reaction (Gao et al. 2020).
-
Self-activating agents include potassium gluconate, ferric ammonium citrate, potassium gluconate, sodium gluconate, sodium alginate, sodium tartrate, zinc citrate, potassium citrate, trisodium citrate, and a combination of citrate salts. Fuertes et al. reported that using sodium alginate, potassium gluconate, sodium citrate, and sodium gluconate as the carbon source and self-activating agent led to produce highly porous activated carbons with surface area ranging from 660 to 1040 m2/g (Fuertes et al. 2014). In another study, Dai et al. produced a porous carbon sheet using potassium acetate as both precursor and self-activating agent (Dai et al. 2018). The production of activated carbon using self-activating agents would solve the problems observed in chemical activation routes, such as the equipment corrosion and generating unwanted secondary pollution.
Physicochemical activation
The physicochemical activation integrates both physical and chemical processes, implying that carbon precursors are first chemically impregnated with activating agents before being physically activated in the presence of oxidizing gas. The advantages of physicochemical activation include its superior surface modification and controlled textural character. The complexity and high energy demands of physicochemical activation would limit its wide application.
Synthesis of advanced carbon materials from biomass
Numerous investigations on activated carbon materials with various zero-dimensional (0D) fullerene, one-dimensional (1D) carbon (CNTs and carbon fibers), two-dimensional (2D) carbon (graphene, graphene oxide, and reduced graphene oxide), and three-dimensional (3D) graphene skeleton have been conducted (Gong et al. 2016, Ovais et al. 2022). Earlier studies on the synthesis of advanced carbon materials applied different methods, such as laser ablation system, chemical vapor deposition, and arc-discharge technique with the employment of catalysts like Co, Ni, and Fe and also by utilizing non-renewable carbon sources such as benzene and other external precursor carbon gases like ethylene and methane (CH4) that are generated from petroleum and coal products (C2H4). However, these procedures have certain restrictions as these nanostructures are made using non-renewable precursors, resulting in toxic waste and adverse environmental effects. Consequently, technologies must be improved for a non-toxic, more viable, and low-cost production of carbon nanostructures obtained from an eco-friendly environment. Biomass’s high carbon content, sustainability, and availability have made it a promising feedstock for producing value-added chemicals and sustainable carbon products.
A lot of research studies have demonstrated that lignocellulosic biomass is an appropriate bio-feed for producing nano-advanced carbon materials such as CNFs and CNTs. It comprises a substantial quantity of cellulose, which several studies have determined to be the precursor for developing CNTs (Sevilla and Fuertes 2010; Omoriyekomwan et al. 2019; Teo and Wahab 2020). Joy et al. proposed a mechanism for the transformation of biomass to CNTs via microwave-assisted pyrolysis; D-glucopyranose, a monosaccharide produced by the breakdown of cellulose, served as a carbon source for the production of CNTs (Omoriyekomwan et al. 2019). Anhydrides, oligosaccharides, and levoglucosan are produced when the glycosidic connections in D-glucose are broken (Zhang et al. 2011), and sugars are further broken down, fissured, and rearranged (Cho et al. 2010). The cleavage of the C–O bonds in levoglucosan is proceeded by aromatization to create layers that resemble graphite, as shown in Fig. 4 (Collard and Blin 2014; Muley et al. 2016). Table 4 shows selected studies on the production of advanced carbon materials from different biomass wastes.
Reaction pathways during the decomposition of cellulose; copyright 2023 Elsevier (Omoriyekomwan et al. 2021)
Several studies report that the inorganic elements co-existing in biomass can catalyze the production of CNT or MWCNT products. By illustrating a single-step synthesis strategy to generate MWCNT, Ramya Araga et al. have successfully established the importance of minerals contained in biomass as catalysts (Araga and Sharma 2017). This method for creating CNTs on the surface of coconut shell-derived carbon does not require extra impregnation of metal nanoparticles. Zhu et al. reported that the use of Mg2SiO4 is the reason behind the nucleation and growth of CNTs (Zhu et al. 2012). The same results were shown by Shi et al. demonstrating the effect of SiC in the nucleation of MWCNT without the need for any additional catalysts (Shi et al. 2014).
Adsorption of pollutants in water phase
Removal of organic pollutants from water
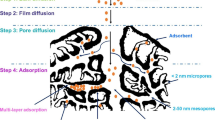
Rapid industrial and agricultural activities have resulted in the discharge of organic pollutants, such as dyes, persistent organic pollutants (POPs), polycyclic aromatic hydrocarbons (PAHs), pharmaceuticals, pesticides, and industrial chemicals into the aquatic environment. Indeed, the adsorption process is a general approach to physically remove these compounds from water and wastewater (Galloni et al. 2022a, b; Galloni et al. 2022a, b). Activated carbon is recognized as one the most widely applied adsorbent for the adsorption of organic pollutants during large-scale applications. Numerous researches on the conversion of biomass-based wastes into carbon materials have been published over the past few decades, along with various surface modification techniques to improve selectivity and adsorption yield (Nor et al. 2013, Arun et al. 2021; Jjagwe et al. 2021; Shukla et al. 2021). In terms of mechanistic pathways, several adsorptive routes have been reported that in general depend on the surface chemistry of the adsorbent (functionalization, wettability, porosity, surface area, and so on), the nature of the organic pollutants and also the working conditions, i.e., pH of the solution. Overall, five adsorption pathways could be classified as shown in Fig. 5.
-
Physical pore filling route: the accumulation of organic molecules inside the pores of biomass-based absorbent is the standard way to remove organic pollutants from water. In this regard, the produced activated carbon must have a high porosity. Additionally, the relationship between the size of the pores and of the organic pollutant is crucial.
-
Electrostatic interaction–based adsorption is based on the attraction or repulsion between the ionic surface of the adsorbent and the ionizable organic molecules. The surface charge and organic pollutants are important components in this scenario. The formation of repulsion forces is caused by the same sort of charge, while opposing charges enhances the fixation of organic pollutants. In this adsorption process, the pH of the solution, which may directly influence the ionic state of the adsorbent surface and organic molecule, is important because acidic or basic modification of the adsorbent surface greatly increases the electrostatic attraction.
-
Hydrogen bonding–based adsorption: this adsorption process is based on the intermolecular or intramolecular interactions between hydrogen and other atoms, such as nitrogen, oxygen, chlorine, fluorine, and bromine. Similar to electrostatic attraction–based adsorption, carbon materials with abundant oxygen functional groups promote the interaction with hydrogen, resulting to adsorption by hydrogen bonding. Additionally, this interaction makes it simple to fix highly polar organic contaminants on the adsorbent surface.
-
Hydrophobic-based adsorption: this mechanism correlates both electrostatic attraction and distribution. The fixation of hydrophobic organic pollutants occurs mostly on the surface of hydrophobic adsorbents. Therefore, the modification of biomass-based carbon materials with hydrophobic agents is commonly used.
-
π–π binding–based adsorption: aromatic organic pollutants with C–C conjugation can form π–π interactions with aromatic structures present in some carbon materials. Oxygen, nitrogen, or amine-based groups can boost π–π interactions.
Common mechanisms of organic pollutants adsorption on the surface of carbon materials, reproduced with permission from Qiu et al. (2022); copyright 2023 Elsevier
Based on the above discussion, the adsorption of organic pollutants is dependent on a number of variables, including the surface chemistry and textural porosity on the one hand, and the properties of the organic contaminants and working circumstances on the other. It is important to note that the adsorption of organic pollutants cannot be accomplished by a single process, but rather through a combination of the several mechanisms mentioned above, with one or two mechanisms predominating.
Table 5 lists the adsorption capacities of carbon materials prepared from different biomass substrates to remove various organic pollutants.
Surface modification to aid the fixation of organic pollutants has frequently been reported. Adsorbent surface hydrophobicity alteration is one method for solving the co-adsorption of water molecules, which typically lessens the fixation of organic contaminants. Sun et al. (2019) modified sunflower biomass–based activated carbon with polydimethylsiloxane (PDMS) to increase the hydrophobicity (Fig. 6a). Study also suggests that the incorporation of porous materials, such as MOFs, could provide a hierarchically structured surface to the biomass-based activated carbon to enhance the adsorption capacity and the selectivity towards the organic pollutants. For instance, Zhao et al. (2020) modified raw kapok fibers biomass by MIL-53 before carbonization to obtain hierarchically structured activated carbon (Fig. 6b) for the adsorption of oils and organic solvents in water. MOF-modified biomass displayed superhydrophobic features compared to raw biomass based on the contact angle results. Similarly, the novel biomass-C@MIL-53-C exhibited superior adsorption capacity that is reportedly 119 times higher than bare biomass-C towards the removal of oils/organic solvents, including n-hexane, chloroform, and so on. Carbon aerogel prepared from winter melon was reported to have high adsorption of oil in the water system (Fig. 6c) (Li et al. 2014). Surface modification of biomass by kaolinite clay was reported by Olu-Owolabi et al. (2017) for the adsorption of 2,4,6-trichlorophenol in water. It was noticed that the surface area decreased by 57% compared to bar kaolinite; however, the ion-exchange capacity increased by fourfold. The adsorption capacity increased by up to 250% compared to bare kaolinite due to the presence of functional groups, proving more electrostatic interactions with the pollutant.
a Modification of sunflower-based activated carbon with PDMS for enhanced hydrophobicity towards the selective adsorption of organic and oily pollutants over water molecules, diesel oil separation from (dyed with red oil O)/water mixture (left 1–3), reproduced with permission from Sun et al. (2019). b Steps of the synthesis of biomass-C@MIL-53-C for the adsorption of oily pollutants in water, reproduced with permission from Zhao et al. (2020). c Valorization of winter melon as carbon aerogel for the oil adsorption in water, reproduced with permission from Li et al. (2014)
Magnetic reusable activated carbon made from biomass has drawn a lot of interest as absorbent recovery is one of the challenges associated with suspension-based adsorption treatment. The application of magnetic materials facilitates the recovery of suspension by applying an external magnetic field. Therefore, recycling the adsorption suspension would be easy (Mehta et al. 2015; Tamjidi et al. 2019). A number of methods, including co-precipitation, hydrothermal, thermal decomposition, sol–gel, chemical reduction, pyrolysis, polyol, and microwave-assisted pyrolysis, have been described to combine magnetic iron with biomass-based carbon materials as reviewed by Noor et al. (2017). Sirajudheen et al. reported the synthesis of MnFe2O4 Moringa oleifera seed–based carbon material for the adsorption of organic dyes (Sirajudheen et al. 2021). For this, firstly Moringa oleifera seeds were carbonized, and then, the produced activated carbon was coated by MnFe2O4 through a simple co-precipitation route. The authors report a significant adsorption capacity that was obtained by magnetic adsorbent as compared to barely activated carbon adsorbent and MnFe2O4.
Removal of heavy metals from water
Over the years, biomass-based activated carbons have been widely studied as sorbents for the selective removal of several heavy metal ions. In particular, different modification processes (i.e., magnetization, oxidation, functional group grafting, and construction of composite inorganic materials) are recognized to be effective strategies for enhancing the adsorbent capacity of activated carbons (Abdolali et al. 2015; Yang et al. 2019; Šoštarić et al. 2020; Shukla et al. 2021). However, the adsorption capacity of activated carbons is driven by different adsorption mechanisms (Fig. 7a), categorized into five classes namely, (i) physical adsorption, (ii) ion exchange, (iii) electrostatic interaction, (iv) surface complexation, and (v) precipitation.
a Adsorption mechanisms of carbon-based materials for heavy metal removal. b Dissolution of biomass and reconstruction in ionic liquid to fabrication of effective bioadsorbent for heavy metal adsorption, reproduced with permission from Zhong et al. (2012)
Physical adsorption, which is independent of the holding power of the chemical bonds, leads the distribution and dispersion of heavy metal species into the pores of the adsorbent material. In fact, the pore size distribution and surface area of the adsorbent play a major role in this adsorption. In particular, the higher the surface area and the mesopores, the quicker the pollutant dispersion onto the adsorbent surface with consequent enhancement of the whole adsorption phenomenon (Ma and Wang 2020; Hoang et al. 2022). Ion exchange is a process during which the exchange of ions between the functional groups containing oxygen species (e.g., –OH, –COOH) and divalent metal cations (M2+) happens. In particular, it is known as the primary adsorption phenomenon between heavy metal species and activated carbons (Yang et al. 2019; Hoang et al. 2022). Electrostatic interaction has been recently classified as another adsorption mechanism, as reported by Yang et al. (2019). In particular, it occurs when the adsorbent’s surface is negatively or positively charged. In this way, an electrostatic attraction force is created, favoring the oppositely charged metal ions towards the adsorbent material. It is crucial to note that the surface properties of carbonaceous materials affect the occurrence of various charges; hence, the pH value and the isoelectric point (pHpzc) are paramount parameters to evaluate (Wang et al. 2014). Surface complexation of different functional groups consists in the interaction between the functional groups (e.g., –OH, O2−, –CO–NH–, and –COOH) of the adsorbent and the heavy metal ions, generally M2+ or complexes (i.e., Pb(OH)+, Cd(OH)+) (Li et al. 2017). In this way, multi-atom structures are formed as a consequence of this phenomenon (Li et al. 2017). Eventually, precipitation or coprecipitation results in formation of solidified participles as a consequence of interactions between heavy metal ions and the adsorbent surface. The process typically occurs in an aqueous solution containing high levels of heavy metal concentrations (Liu et al. 2020).
The reduction process is crucial for the extraction of heavy metals when there are high-valence heavy metal ions present. Heavy metal ions are, in fact, initially reduced to low-valence states before being eliminated by surface complexation or ion exchange pathways (Li et al. 2017). The above-described mechanisms resume all the possibilities in which carbon-based materials can remove heavy metal ions. The physicochemical characteristics of the adsorbent, the properties of heavy metal ions, and the experimental settings in which the adsorption phenomenon occurs all have a significant role in how effective the adsorption capacity is in this context (e.g., pH, initial concentration of heavy metal ions, presence of coexisting ions, temperature).
To improve the adsorption ability, most of the studies report surface modification by chemical agents to functionalize the surface for enhanced fixation of heavy metal ions through the mechanistic pathways reported above. Li et al. (2022a, b) modified Medulla Tetrapanacis biomass with nitrogen (from urea) and NaHCO3 during its pyrolysis at 700 °C. N-rich biochar showed excellent sorption capacities of 468.5 mg/g and 1466.5 mg/g for Cu2+ and Pb2+, respectively. Heavy metal fixation occurs through mechanistic mechanisms such physical adsorption, complexation, and precipitation. Citric acid is applied to the surface of biomass-based adsorbents to enrich the surface with oxygenated functional groups, which enhances the fixation of heavy metal cations (Monroy-Figueroa et al. 2015). For the fixation of heavy metal cations, adsorbents with negatively surfaces can promote the fixation ability. Ali Khan et al. (2017) developed a dodecyl sulfate chain-anchored mercerized lignocellulosic biomass, which enhances the electrostatic interactions with heavy metal cations. The authors report a very rapid fixation of Pb2+, Cd2+, and Zn2+ on the negatively charged biomass. Lignocellulosic biomass can be broken down and rebuilt to improve its sorption capacity. For example, Zhong et al. have dissolved milled bamboo biomass in IL 1-ethyl-3-methylimidazolium acetate and then reconstructed it in ionic liquid (saturated sodium bicarbonate), to build cross polymers, as shown in Fig. 7b. The authors reported that the maximum adsorption capacities of Pb2+ and Cd2+ were 381.7 and 278.6 mg/g, wherein an ion exchange process was the adsorption mechanism on the surface of bio adsorbent.
Adsorption of pollutants in air phase
Carbon materials are widely used to remove several air pollutants through adsorption (Raso et al. 2014; Ligotski et al. 2019; Wang et al. 2020a, b, c, d, e, f; Roegiers and Denys 2021; Maximoff et al. 2022). Recently, the valorization of biomass-based materials into activated carbon or biochar towards the adsorption or/and reduction of air pollutants, including VOCs, NOx, CO2, and so on, has been extensively studied. This section discussed biomass-based activated carbon materials for air treatment by stressing modification and activation routes.
VOC adsorption
VOCs are primarily discharged from industries, such as organic chemicals, printing, and paint, with considerable adverse effects on the environment (Wang et al. 2013; Liu et al. 2019a, b). The adsorption of VOCs on activated carbons depends on different factors, including the surface area, pore size, and the surface functional groups from one side, and characteristics of volatile molecules such as polarity, weight, and hydrophobicity (Lucena et al. 2012; Huang et al. 2021; Rahbar-Shamskar et al. 2022). Biomass-based activated carbon materials were used as adsorbents to capture VOC materials (Ikram et al. 2022). The improved surface function enables greater chemical interactions, surface modification is a crucial role in enhancing VOC fixation. Shen et al. (2020) reported that the activation of corncobs-derived carbon adsorbent with KOH improves the selectivity of VOC adsorption (851.3 and 854.9 mg/g for benzene and toluene, respectively) three times better than the adsorption capacities using a commercial activated carbon. Li et al. (2011) studied the chemical modification of coconut shell–based activated carbon towards the fixation of hydrophobic VOC (o-xylene). It was reported that the acid modification, using sulfuric acid and phosphoric acid, decreased the adsorption capacity of o-xylene. In contrast, the modification by alkalis (ammonia and sodium hydroxide) boosts the adsorption of o-xylene.
The applied pressure during the adsorption of VOCs by activated carbon-based materials has a significant role depending on the pores size. Ma et al. (2021) developed biomass-based hierarchical porous carbon with highly surface area of 3936 m2/g via KOH-combined pyrolysis. The adsorption of VOCs (acetone and methanol) under high pressure, according to the authors, is controlled by the overall pore volume. The adsorption of VOCs is also controlled by surface oxygen, functional groups, and electrostatic interactions at low pressure. Another important consideration is the VOC molecule’s structure. It was reported by Li et al. (2022a, b) that the micropores and heteroatom influence the oxygenated VOCs adsorption behavior on biomass-based hierarchical porous carbon at low pressure. However, the adsorption of aromatic-based VOCs depends on different sizes at various applied pressures, as summerized in Table 6.
NOx adsorption and reduction
Nitrogen oxides (NOx) seriously affect human health and the environment. For decades, NOx pollution has been considered one of the environmental issues that requires an urgent investigation to develop approaches for their removal or/and reduction (Sun et al. 2016; Damma et al. 2019; Zhang et al. 2020a, b). Removal of NOx via adsorption using biomass-based activated carbon materials has been a hot topic recently (Reza et al. 2020). Wang et al. (2010) investigated the effect of biomass type (wheat straw, char of wheat straw, cotton stalk, and rice husk) on the reduction of NOx during the re-burning of these wastes. Consequently, the heterogeneous reduction of NO reached approximately 59% total NOx. Their results also showed the different biomass behaved differently toward reducing NOx. For instance, out of the different biomass fractions, NO reduction followed the order cotton straw > wheat straw > rice husk. Hence, the appropriate selection of biomass is essential for effectively removing NOx. Other studies have shown that using biomass or coal as re-burning fuels could heterogeneously reduce NO using pyrolytically produced char at high temperatures (Zhong et al. 2002; Zhang et al. 2007; Guo et al. 2019). Shu et al. (2018) investigated a series of herbaceous and lignocellulose biomass as activated carbon support for the subsequent reduction of NOx to N2. The authors examined the effect of carbonization temperature, biomass type, and catalyst composition on NOx reduction. They reported that NOx adsorption onto carbon was higher at a temperature lower than 250 ºC in all materials. Although enhanced reaction adsorption at low temperatures is achievable, high flue gas temperatures and low levels of acidic gas can present a major bottleneck, resulting in low adsorption capacities and poor selectivity (Abdulrasheed et al. 2018). Modifying the surface of activated carbon is one way to help increase the formation of immobilized functional groups that improves NOx removal from air. For example, the selective reduction of NOx at higher temperatures could also be achieved via the addition of an alkali, such as KOH (Sumathi et al. 2010), transition, and alkaline-earth metals such as potassium (García-García et al. 1997; Illán-Gómez et al. 1998), calcium (Kordylewski et al. 2005), and nickel (Illán-Gómez et al. 1999). For instance, by impregnating a coconut shell-activated carbon with KOH, Sumathi et al. (2010) achieved a simultaneous reduction of both NOx and SO2. Furthermore, the AC could access the pore structure after adding potassium, significantly enhancing the efficiency of NOx and SO2 reductions due to the high reactivity on its surface.
The promotional effect of oxygen on the adsorption of NOx when using activated carbon as a catalyst has been studied previously. Zhang et al. (2008) examined NO adsorption by activated carbon in the presence or absence of oxygen in a reaction having 500 mg/L NO and a temperature below 100 °C. Interestingly, a negligible NO reduction was recorded in the system without oxygen, but about 10% reduction was obtained with oxygen. The role of activated carbon is to first convert NO to NO2 that could readily be adsorbed. The catalytically oxidizing NO to NO2 could be a practical way of removing NOx from flue gas since NO2 is readily removed by water. Loading an activated carbon with silver nanoparticles was a successful route to enhance the adsorption of NO2, promoting its conversion to NO and the subsequent conversion to N2 (Seredych et al. 2010). Additional tests using fed dry air containing 0.1% NO2 at room temperature showed that 0.40 mmol/g of NO2 was adsorbed.
CO2 adsorption
Over the past few decades, with the continuous rise in CO2 concentration, a common greenhouse gas has been a source of serious environmental threat by serving as a heat nesting site in the atmosphere leading to global warming. A common method for fixing CO2 is adsorption. The yield of adsorption depends on its thermodynamic. It could be fixed on the surface through physisorption or chemisorption (Creamer et al. 2014; Lee and Park 2015). In physisorption, CO2 is fixed through weak van der Waal forces, whereas chemisorption occurs via stronger chemical bonds, which lead to higher adsorption capacity, but the regeneration is more difficult. Biomass-based activated carbon materials have been widely used for CO2 capture (Abuelnoor et al. 2021). Similar to VOCs and NOx, CO2 adsorption depends on the materials’ textural characteristics and functional groups and the working conditions, such as the pressure and temperature. To improve the selectivity of CO2 adsorption on activated carbon, studies have revolved around the synthesis and functionalization of activated carbon from different biomass materials. Manyà et al. (2018) prepared biochar from vine shoots through CO2-physical and KOH-chemical routes. The authors reported that the chemically activated biochar exhibits maximum adsorption of CO2 at 25 °C at an absolute pressure of 15 kPa, whereas the physical activation with CO2 shows maximum adsorption at 75 °C. In addition, these biochar adsorbents showed enhanced selectivity towards CO2 over N2. Another study, which compares the physical and chemical activation of biomass (spent coffee grounds) towards CO2 adsorption, was reported by Plaza et al. (2012). It was found that the KOH chemical activation led to the best CO2 uptake, whereas the CO2 physical activation leads to better CO2/N2 selectivity. Although surface area is a crucial component of CO2 adsorption, several studies have shown that increasing surface area is not the sole way to enhance CO2 fixation. Wang et al. (2012) prepared an activated carbon with an excellent surface area of 3404 m2/g via the valorization of celtuce leaves waste into activated carbon using air-drying, pyrolysis, and chemical activation by KOH. However, the fixation of CO2 was 6.04 and 4.36 mmol/g at 0 and 25 °C. This result is not very far from activated carbon-based materials with much lower surface area (Abuelnoor et al. 2021).
The functionalization of activated carbon-based materials to enhance the fixation of CO2 is an integral approach (Arenillas et al. 2005; Pevida et al. 2008; Lee and Park 2015). In general, acidic, basic, or neutral heteroatoms are incorporated on the surface to modify their electronegativity and provide active sties for enhanced attraction of CO2 molecules (Shafeeyan et al. 2010). Figure 8 summarizes the most common surface modification of activated carbons by oxygen or nitrogen functional groups. For example, incorporating nitrogen into the surface of activated carbon could boost the fixation of CO2. Zhang et al. (2016a, b) concluded that the heat treatment of KOH-activated black locust with ammonia leads to the incorporation of nitrogen on the surface, which in turn boosts the fixation of CO2 as compared with bare KOH-activated black locust. Based on the surface functional groups and pollutants characteristics, different surface interactions or repulsion would take place as shown in Fig. 9.
Common surface modification of activated carbon-based materials for boosting the fixation or air pollutants; a oxygen-based group modification, b nitrogen-based groups modification, reproduced with permission from Abdulrasheed et al. (2018)
Types of interactions between air pollutants and activated carbon surface as a function of the symbol of the second largest eigenvalue (λ2) and ρ are the electron density, reproduced with permission from Wang et al. (2022)
SO2 adsorption
The emission of SO2, a toxic gas, is a severe environmental issue nowadays. A lot of efforts have been made for the appropriate removal/reduction of SO2 in phase using different technologies, namely wet scrubbing (Zhao et al. 2021a, b), dry-based reduction technologies (Ng et al. 2022), and adsorption (Raymundo-Pinero et al. 2000; Kang et al. 2020; Liu et al. 2021). The valorization of biomass into activated carbon towards the reduction of SO2 removal was investigated by several studies. Illingworth et al. reported the synthesis of activated carbon from fibrous waste biomass (flax) for SO2 adsorption in a continuous-flow reactor (Fig. 10a) (Illingworth et al. 2019). The surface area increased with the carbonization temperature, which was found to be 126 and 1177 m2/g at 450 and 800 °C, respectively. In addition, a very microporous surface was obtained. The adsorption of SO2 increases with the surface of the materials, whereas the results showed that flax-based activated carbon has better adsorption than the commercial one. Wang et al. (2022) prepared an activated coke from valorizing mixed sewage sludge and waste biomass via carbonization and ammunition for SO2 adsorption. It was reported that the nitrogen-containing functional groups boost the chemical fixation of SO2. The presence of inorganic components in biomass-based materials can provide further reduction reactions on top of the adsorption. Zhang et al. (2020c) synthesized nitrogen-enriched biochar from different biomass materials and reported an adsorption capacity of up to 216.19 mg/g at 120 °C. Xu et al. (2016) prepared three types of biochar from different starting materials, including rice hush, dairy manure, and sewage sludge for the adsorption of SO2 from the air. The biochar-based materials showed adsorption capacities of about 8.87–15.9 mg/g. However, the increase of humidity content from 0 to 50% led to a threefold increase in adsorption capacities due to the formation of alkaline water functional groups to boost the fixation of SO2. Interestingly, it was claimed that biochar with higher mineral constituents led to better SO2 removal. As shown in Fig. 10b, mineral constituents can convert SO2 into SO42− that is converted into different mineral salts.
Mercury adsorption
Biomass-based activated carbon materials were also used for the fixation of gaseous Hg0 (Skodras et al. 2007; Asasian et al. 2012; Egirani et al. 2021). Liu et al. reported the valorization of seaweed biomass into activated carbon for the adsorption of Hg0 in the air (Liu et al. 2019a, b). It was found that the fixation is controlled by mass transfer at 80 °C, whereas the increase in the temperature to 120 and 160 °C boosts the chemisorption. The high surface area and porosity aided the physisorption reaction. The investigation also proves that C–Cl and oxygen functional groups on the activated carbon surface promoted the chemisorption reaction. In another study [227], corn cob activated carbon was activated using ZnCl2. It was reported that both Zn and Cl, besides to enhance porosity and surface area, play an essential role in capturing of Hg0. Cl could boost the surface oxidation of Hg0 into its oxides, while Zn can increase tensile strength and water adsorption. Zhao et al. reported that the efficiency of Hg0 adsorption on the surface of biomass carbon and activated carbon depends on the pore structure and Br content (Zhao et al. 2021a, b). It was claimed that C–Br could oxidize the physically adsorbed Hg0 into HgBr2, then transform it to C–O–Hg, and finally desorb it as HgO. At temperatures ranging from 80 to 120 °C, the oxidation of the surface of biomass carbon is more pronounced. At the same time, further increase leads to an inhibited oxidation due to the breakdown of Van der Waals forces bonds. The bromination of biomass ash towards the enhanced usage of a chemical–mechanical approach was reported by Bisson et al. (2013). Arvelakis et al. (2010) studied the pretreatment role and biomass activation. The authors concluded that the untreated biomass exhibits better Hg0 adsorption than the lignite-activated carbon treated at 850 °C. It was claimed that the high calcination temperature leads to the decomposition of alkali groups, reducing the capture of Hg0. In addition, it was mentioned that the high surface area of the activated carbon does not seem to have a significant effect on the adsorption of Hg0.
In terms of surface oxygen, both yield of atomic oxygen on the surface and nature of oxygen groups are determinants for the fixation of Hg0. Shen et al. studied oxygen groups’ role in the adsorption of Hg0 on the surface of biomass-based activated carbon (Shen et al. 2018). The treatment of the adsorbent surface via non-thermal plasma incorporated oxygen functional groups on the surface. The authors reported that carbonyl and ester groups boost the fixation of Hg0, while epoxy, carboxyl, and hydroxyl groups reduce the adsorption capacity (Fig. 11). The adsorption was improved after the non-thermal plasma treatment of the activated carbon due to the incorporation of ester and carbonyl groups. The promotion effect of the adsorption ability is due to the higher adsorptive energies of Hg0 on the carbonyl and ester groups than on the surface of activated carbon. At the same time, the suppression is because of the lower adsorption energies of Hg0 on the epoxy, carboxyl, and hydroxyl groups compared to that on the surface of activated carbon.
Adsorption energies of Hg.0 on different oxygen groups, reproduced with permission from Shen et al. (2018)
Photocatalytic removal of organic pollutants from water
Lignocellulosic biomass–based materials have been combined with many photocatalytic active materials, displaying synergistic effect between adsorption and photocatalysis. Indeed, carbon materials have been regarded as successful supports for photocatalysts because of their structure and physical stability (Baek et al. 2013; How et al. 2014; Mian and Liu 2018; Zhang et al. 2018a, b). Several mechanism pathways and eco-benefits can be obtained when the photocatalytic nanoparticles and lignocellulosic biomass are combined (Fig. 12a), as follows:
-
Adsorb and shuttle process: In such kinds of materials, a systematic cycling process of adsorption followed by photooxidation occurs. The sorption area concentrates the organic pollutants near the photoactive area for enhanced ROS attack (Djellabi et al. 2020a, b, c, Saber et al. 2021; Abderrahim et al. 2022b). In naked-based photocatalysts, the photocatalytic kinetics is very slow because of low adsorption ability, and as a consequence, most of produced ROSs will not be very interactive with organic pollutants (Ollis 2018, Wang, Zhang et al. 2020a, b). If we use a naked photocatalyst with high adsorptive ability, the surface of the photocatalyst would be covered, and no further light penetration and photocatalytic activity would occur. In a system where a photoactive area exists on an adsorptive area, the concentration of pollutants would not affect the photoactivity of uncoated photoactive nanoparticles.
-
Enhanced visible light response: The hybridization of photocatalysts with carbon-based materials leads to improved photoactivity under visible light through different mechanisms, including (i) photosensitizing, where the biomass polymer could absorb the light and transfer its energy to the photocatalyst, and (ii) decrease in band gap because of the surface interaction during the synthesis, such as the formation of bridge bonds. Djellabi et al. proved the formation of Ti–O–C in TiO2/biomass-based composite (Djellabi et al. 2019a, b, c, d). In addition to the enhanced light absorption, an excellent charges separation would occur through this bridge (Zhang et al. 2020a, b).
-
Enhanced adsorption ability: As discussed above, the adsorb and shuttle process is the primary mechanism for the oxidation of organic pollutants; the sorption ability also helps decrease the number of contaminants in water and contributes to fixing the generated toxic by-products (Djellabi et al. 2021a, b, c).
-
Self-generation of the materials: In general, adsorption suffers from the fast deactivation of the adsorbent because if there is an accumulation of pollutants on the surface. However, in photocatalytic/adsorptive composites, the photocatalytic action plays the role of surface cleaning, wherein ROSs decrease the amount of the contaminants on the surface, allowing a longer lifetime of the photocatalyst.
-
Easy handling and recovery: Naked photocatalytic nanoparticles are difficult to handle because of the nanosized particles. After the water treatment, it is tough to recover the particles from water, and a membrane separation step is required, increasing the process cost (Friehs et al. 2016; Djellabi et al. 2021a, b, c). However, the recovery and handling of NPs/biomass composites are easier through the simple decantation and filtration.
a Scheme shows the main benefits of combining biomass and photocatalytic nanoparticles. b Synthesis process of magnetic TiO2-biomass for water purification. c Photocatalytic mechanism on the surface of TiO2/Fe3O4 and TiO2/Fe3O4@biomass composites; b and c were reproduced from Djellabi et al. (2019a, b, c, d); copyright 2023 Elsevier
Several biomass-supported photoactive composites have been developed via several synthesis methods using different raw biomass supports and semiconductors. TiO2 hybridized with biomass-based materials has received the most attention (Kim and Kan 2016; Khataee et al. 2017; Zhang et al. 2017; Djellabi et al. 2019a, b, c, d; Lu et al. 2019; Fazal et al. 2020). Djellabi et al. (2019a, b, c, d) prepared magnetic TiO2-Fe3O4@biomass-based composite to oxidize organic pollutants in water. The synthesis process is shown in Fig. 12b. The authors explained the role of biomass in TiO2-Fe3O4@biomass as compared to bare TiO2-Fe3O4. Based on many reports, TiO2-Fe3O4 does not build a successful heterojunction due to the intense recombination of electrons/holes charges, wherein Fe3O4 plays the role of a recombination center (Beydoun et al. 2000; Abramson et al. 2009; Salamat et al. 2017). Zielińska-Jurek et al. reported the dissolution of Fe3O4 because of the electrons coming from the conduction band of the TiO2. To isolate TiO2 and Fe3O4, biomass was used to support both TiO2 and Fe3O4; meanwhile, biomass can play the role of electron acceptor via the Ti–O–C bridge, avoiding the transfer of dissolution of Fe3O4 (Fig. 12c). TiO2-Fe3O4@biomass can be quickly recovered by the magnetic field. Several studies on g-C3N4 supported on biomass materials were previously reported (Pi et al. 2015; Jeon et al. 2017; Kumar et al. 2017, 2018). Sun et al. said that C3N4/ferrite/biochar, on top of its photoactivity, can initiate Fenton or/and photo-Fenton reactions further to boost the oxidation of organic pollutants (Sun et al. 2020). Zhou et al. reported porous lignocellulose-derived Juncus effusus fiber–supported photocatalysts for efficient wastewater treatment (Zhou et al. 2022). The exquisite 3D microstructure of lignocellulose-derived Juncus effusus fiber and affluent •OH group enable it to support some catalyst powders such as g-C3N4. The lignocellulose-derived fiber-supported photocatalysts showed excellent photodegradation performance for different organic pollutants, such as organic dyes and antibiotics. Roa et al. (2021) clarified the photodegradation mechanism of lignocellulose-based material via the dye removal tests and pointed out that most of these lignocellulose-derived carbon materials possess rich pore structures and surface functional groups, which make them to show strong enrichment capacity for pollutants as base materials, making up for the inability of many powder photocatalysts to quickly capture pollutants. The enhanced structural property and presence abundant functional group greatly improved the ability of photocatalytic degradation of pollutants. ZnO/biomass-based composites for the oxidation of different organic pollutants have also been widely reported (Leichtweis et al. 2020; Yu et al. 2021; Cai et al. 2022). A decrease in the band gap in ZnO/biochar composites was reported by Gonçalves et al. (2020). The authors suggested that the reduction of e_/h+ recombination at ZnO/biochar composite; also, the sample calcined at 400 °C showed the highest production of ROSs. Other photocatalysts were coated as well on biomass-based materials. For example, BiOBr and BiOCl combined with biochar lead to improve visible light responsiveness, as discussed by Li et al. (2016a, b). Chen et al. developed magnetically recoverable biochar@ZnFe2O4/BiOBr Z-scheme by solvothermal, and the authors report that BiOBr boosts the separation of charges and the adsorption.
Photocatalytic removal of heavy metals from water
The photocatalytic reduction of heavy metals by photocatalysts supported on lignocellulose biomass materials has been widely reported and discussed (Wang et al. 2020a, b, c, d, e, f; Bhavani et al. 2022; Lu et al. 2022). The photoproduced electrons achieve the reduction of heavy metals by photocatalytic means on the conduction band of the photocatalyst and some reducing organic agents that could be produced from the oxidation of co-existed organic molecules, i.e., CO2•− (E0(CO2/CO2•−) ≈ − 2.0 V (Marinho et al. 2017). In addition, some ROSs, such as −•O2, can participate in reducing some heavy metals (An et al. 2020a, b). From the environmental viewpoint, reducing heavy metals to the metallic state, or in some cases to lower oxidation states, i.e., Cr(VI) to Cr(III), could reduce their toxicity and mobility in water. In addition, during the photocatalytic reduction, photodeposition occurs at the surface of the photocatalyst to produced and extract metallic species (Djellabi et al. 2016). However, the photodeposition process has been regarded as inconvenient in many reports as it deactivates the surface and blackens the light penetration/excitation processes (Zhang et al. 2018a, b). It is worth mentioning that, in general, the photocatalytic reduction of heavy metals accompanies the oxidation of organic molecules as hole scavengers (Djellabi et al. 2021c). The presence of hole scavenger molecules promotes the separation of electrons on the conduction band and limits the re-oxidation of produced metallic species (reverse reaction) by ROSs, as discussed in depth previously (Djellabi and Ghorab 2015; Djellabi et al. 2020a, b, c; Bortolotto et al. 2022). Many physical and photonic mechanisms can occur in terms of the photocatalytic reduction of heavy metals by photocatalytic NPs/biomass-based composites. In terms of TiO2-biomass photocatalysts, Djellabi et al. investigated the mechanistic role of the interfacial link formed between the biomass and TiO2 nanoparticles on the light absorption, band gap, and photocatalytic efficiency for Cr(VI) reduction (Djellabi et al. 2019a, b, c, d). It was claimed that the increase of biomass ratio in Biomass/TiO2 composites leads to decreased band gap due to stronger interfacial interactions. The authors suggested the formation of a Ti–O–C bridge between TiO2 and biomass, as shown in Fig. 13a. Employing FTIR analysis (Fig. 13b), a new peak at 850 cm−1 was produced when TiO2 and biomass were hybridized, and the intensity of the peak is related to biomass content. On top of the decrease in the band gap, enhanced charge transfer and photosensitising could produce more redox species through this bridge. Magnetic biomass-TiO2 for Cr(VI) reduction was also reported, wherein the co-presence of TiO2 and magnetic Fe3O4 boosts the photoreduction of Cr(VI) synergistically under solar light. Several photocatalytic materials have been combined with lignocellulosic biomass-based materials such as g-C3N4 (Li et al. 2022a, b), BiVO4 (Li et al. 2022a, b), Ag3PO4 (Abderrahim, Djellabi et al. 2022a, b), Bi2WO6 (Wang et al. 2020a, b, c, d, e, f), ZnO (Cruz et al. 2020), and photoactive polymers (Djellabi et al. 2020a, b, c; Djellabi et al. 2021a, b, c). On the other hand, the toxicity of some heavy metals can be reduced by their reduction to lower oxidation states, such as in the case of arsenic. In this regard, the oxidation by photocatalytic means is similar to the photooxidation of organic pollutants based on ROSs attack. Yan et al. reported that As(III) could be effectively removed by NiS/NiSe/3D porous biochar through a synergistic adsorption-photocatalytic process (Yan et al. 2021). The adsorbed As(III) can be photocatalytically converted into As(V) by photogenerated ROSs on the surface of biochar. Xue et al. studied the removal of As(III) by SnS2-supported biochar and decorated with heteropoly acid (Xue et al. 2021). The addition of phosphotungstic acid to biochar/SnS2 system improves the removal of As(III) by 1.5 times. TiO2-biochar was also used to remove As(III) by adsorption-photocatalytic system, wherein −•O2 species were the most responsible oxidative ROSs for the oxidation of adsorbed As(III).
a Ti–O–C bridge formed between TiO2 nanoparticles and carbonaceous materials, reproduced with permission from Djellabi et al. (2019a, b, c, d). FTIR spectra of TiO2-biomass samples (OP: olive pit–based biomass), reproduced with permission from Djellabi et al. (2019a, b, c, d); copyright 2023 Elsevier
Similar to the photooxidation of organic pollutants, the photocatalytic removal of heavy metals by biomass supported photocatalysts materials is released through the so-called adsorb and shuttle process, wherein the highly adsorbing domain concentrates metallic species nearby the photocatalytic domain to be reduced/oxidized as discussed in depth previously (Djellabi et al. 2020a, b, c, Saber et al. 2021, Fellah et al. 2022).
Conclusions and challenges
This review summarizes the main applications of valorized lignocellulosic biomass for environmental remediation. Lignocellulose biomass wastes can be converted into activated carbon via several ways and activation processes. The use of some techniques, i.e., microwave carbonization, might lead to obtaining highly activated carbon, having excellent porosity and functionality for enhanced adsorption of water and air pollutants. In addition, the incorporation of photocatalytic nanoparticles on the surface of lignocellulose biomass-based materials aims to solve several challenges associated with the conventional photocatalysis, such as a significant enhancement in the kinetics due to the adsorb and shuttle process, reduction in toxic-by products generation because of the highly adsorptive ability, and the improvement of light absorption and charges separation (interfacial interaction, i.e., C–O–Ti bridge, and photosensitizing). The separation of photocatalyst/carbon composites is quite easier than the recovery of nanosized naked photocatalysts. In addition, the cost of photocatalysts/lignocellulose biomass would be cheaper. From the eco-environmental point of view, the valorization of lignocellulosic biomass into carbon materials to be used as adsorbents for water and air pollutants, or to support photocatalytic particles suits very well the concepts of circular bioeconomy in terms of waste recycling, use bio-resource and sustainability. To better scale up lignocellulose biomass waste for large environmental remediation applications, some challenges need to be addressed. In terms of activated carbon from lignocellulosic biomass, sustainable approaches for the activation and carbonization with the use of harmful chemicals are required to be investigated to avoid secondary water and air pollution, e.g., the production of toxic gas of pyrolysis and use of highly toxic chemical for the activation (highly corrosive agents, i.e., KOH or H3PO4). Self-activation approaches to produce carbon materials instead of chemical or physical routes require more investigation because of its sustainability and low cost.
For photocatalytic applications, certainly, most of the studies reported that synergetic effects take place to promote the removal of pollutants as compared to simple adsorption and photocatalysis primarily due to the adsorb and shuttle process. In addition, the oxidation of organic contaminants on the surface of adsorbents is regarded as a self-regeneration, allowing a more prolonged use of the material. However, up to date, this approach is not considered at large scale because of other technical issues, such as the type of irradiation and photoreactor that allows better light penetration if the black photocatalyst/carbon material is used. Self-oxidation of biomass by photoproduced ROSs should be estimated. Since ROSs could oxidize the biomass, it is highly recommended to check the release of products from the biomass during photocatalytic experiments and the stability of the structure by some surface characterization. Further studies to test adsorbents and photocatalysts should be conducted in real conditions and pilot scale. Techno-economic and sustainability factors should be estimated.
Standardized protocols for carbon materials fabrication from lignocellulosic biomass should be developed based on the type of raw materials and the targeted application. In fact, even though chemical activation leads to highly active activated materials, it would be better to seek for advanced green approaches to design activated carbon–based materials in order to avoid the over usage of harmful and toxic chemicals. Most of research studies have been carried out on the valorization of a selected biomass waste; however, real municipal biomass wastes contain a variety of biomass materials. At a large scale, it is hard to separate different biomass wastes from each other. Therefore, it would be interesting to investigate the conversion of real municipal biomass wastes as a total into carbon materials to widen the valorization of real biomass wastes in to valuable products.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Abderrahim N, Boumnijel I, Amor HB, Djellabi R (2022a) Heat and ZnCl2 chemical carbonization of date stone as an adsorbent: optimization of material fabrication parameters and adsorption studies. Environ Sci Pollut Res 29(30):46038–46048
Abderrahim N, Djellabi R, Amor HB, Fellah I, Giordana A, Cerrato G, Di Michele A, Bianchi CL (2022b) Sustainable purification of phosphoric acid contaminated with Cr (VI) by Ag/Ag3PO4 coated activated carbon/montmorillonite under UV and solar light: materials design and photocatalytic mechanism. J Environ Chem Eng 10(3):107870
Abdolali A, Ngo HH, Guo W, Zhou JL, Du B, Wei Q, Wang XC, Nguyen PD (2015) Characterization of a multi-metal binding biosorbent: chemical modification and desorption studies. Biores Technol 193:477–487
Abdulrasheed A, Jalil A, Triwahyono S, Zaini M, Gambo Y, Ibrahim M (2018) Surface modification of activated carbon for adsorption of SO2 and NOX: a review of existing and emerging technologies. Renew Sustain Energy Rev 94:1067–1085
Abramson S, Srithammavanh L, Siaugue J-M, Horner O, Xu X, Cabuil V (2009) Nanometric core-shell-shell γ-Fe2O3/SiO2/TiO2 particles. J Nanopart Res 11(2):459–465
Abuelnoor N, AlHajaj A, Khaleel M, Vega LF, Abu-Zahra MR (2021) Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 282:131111
Acharya B, Dutta A, Minaret J (2015) Review on comparative study of dry and wet torrefaction. Sustain Energy Technol Assess 12:26–37
Aguayo-Villarreal I, Bonilla-Petriciolet A, Muñiz-Valencia R (2017) Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J Mol Liq 230:686–695
Ahmad F, Daud WMAW, Ahmad MA, Radzi R, Azmi AA (2013) The effects of CO2 activation, on porosity and surface functional groups of cocoa (Theobroma cacao)–shell based activated carbon. J Environ Chem Eng 1(3):378–388
Ahmed MJ (2016) Preparation of activated carbons from date (Phoenix dactylifera L.) palm stones and application for wastewater treatments. Process Saf Environ Prot 102:168–182
Ahsan MA, Jabbari V, Islam MT, Kim H, Hernandez-Viezcas JA, Lin Y, Díaz-Moreno CA, Lopez J, Gardea-Torresdey J, Noveron JC (2018) Green synthesis of a highly efficient biosorbent for organic, pharmaceutical, and heavy metal pollutants removal: engineering surface chemistry of polymeric biomass of spent coffee waste. J Water Process Eng 25:309–319
Ali Khan M, Ali MS, Abdullah Alothman Z, Siddiqui MR, Al-Lohedan HA (2017) Dodecyl sulfate chain-anchored mercerized lignocellulose agro-waste: an effective and sustainable adsorbent to sequester heavy metal ions from an aqueous phase. Environ Prog Sustain Energy 36(6):1676–1684
An D-S, Zeng H-Y, Xiao G-F, Xiong J, Chen C-R, Hu G (2020a) Cr (VI) reduction over Ag3PO4/g-C3N4 composite with pn heterostructure under visible-light irradiation. J Taiwan Inst Chem Eng 117:133–143
An Y, Tahmasebi A, Zhao X, Matamba T, Yu J (2020b) Catalytic reforming of palm kernel shell microwave pyrolysis vapors over iron-loaded activated carbon: enhanced production of phenol and hydrogen. Biores Technol 306:123111
Araga R, Sharma CS (2017) One step direct synthesis of multiwalled carbon nanotubes from coconut shell derived charcoal. Mater Lett 188:205–207
Arami-Niya A, Daud WMAW, Mjalli FS, Abnisa F, Shafeeyan MS (2012) Production of microporous palm shell based activated carbon for methane adsorption: modeling and optimization using response surface methodology. Chem Eng Res Des 90(6):776–784
Arenillas A, Rubiera F, Parra J, Ania C, Pis J (2005) Surface modification of low cost carbons for their application in the environmental protection. Appl Surf Sci 252(3):619–624
Arslanoğlu H (2019) Direct and facile synthesis of highly porous low cost carbon from potassium-rich wine stone and their application for high-performance removal. J Hazard Mater 374:238–247
Arun J, Nirmala N, Priyadharsini P, Dawn S, Santhosh A, Gopinath K, Govarthanan M (2021) A mini-review on bioderived carbon and its nanocomposites for removal of organic pollutants from wastewater. Mater Lett 310:131476
Arvelakis S, Crocker C, Folkedahl B, Pavlish J, Spliethoff H (2010) Activated carbon from biomass for mercury capture: effect of the leaching pretreatment on the capture efficiency. Energy Fuels 24(8):4445–4453
Asasian N, Kaghazchi T, Soleimani M (2012) Elimination of mercury by adsorption onto activated carbon prepared from the biomass material. J Ind Eng Chem 18(1):283–289
Asiltürk M, Şener Ş (2012) TiO2-activated carbon photocatalysts: preparation, characterization and photocatalytic activities. Chem Eng J 180:354–363
Aworn A, Thiravetyan P, Nakbanpote W (2008) Preparation and characteristics of agricultural waste activated carbon by physical activation having micro-and mesopores. J Anal Appl Pyrol 82(2):279–285
Baek M-H, Yoon J-W, Hong J-S, Suh J-K (2013) Application of TiO2-containing mesoporous spherical activated carbon in a fluidized bed photoreactor—adsorption and photocatalytic activity. Appl Catal A 450:222–229
Balahmar N, Al-Jumialy AS, Mokaya R (2017) Biomass to porous carbon in one step: directly activated biomass for high performance CO2 storage. J Mater Chem A 5(24):12330–12339
Barin GB, de Fátima Gimenez I, da Costa LP, Souza Filho AG, Barreto LS (2014) Influence of hydrothermal carbonization on formation of curved graphite structures obtained from a lignocellulosic precursor. Carbon 78:609–612
Bedia J, Peñas-Garzón M, Gómez-Avilés A, Rodriguez JJ, Belver C (2020) Review on activated carbons by chemical activation with FeCl3. C 6(2):21
Bergman PC, Boersma A, Zwart R, Kiel J (2005) Torrefaction for biomass co-firing in existing coal-fired power stations. Energy Research Centre of the Netherlands, ECN-C-05-013
Beydoun D, Amal R, Low GK-C, McEvoy S (2000) Novel photocatalyst: titania-coated magnetite. Activity and photodissolution. J Phys Chem B 104(18):4387–4396
Bhatnagar A, Sillanpää M, Witek-Krowiak A (2015) Agricultural waste peels as versatile biomass for water purification–a review. Chem Eng J 270:244–271
Bhavani P, Hussain M, Park Y-K (2022) Recent advancements on the sustainable biochar based semiconducting materials for photocatalytic applications: a state of the art review. J Clean Prod 330:129899
Bisson TM, Xu Z, Gupta R, Maham Y, Liu Y, Yang H, Clark I, Patel M (2013) Chemical–mechanical bromination of biomass ash for mercury removal from flue gases. Fuel 108:54–59
Bortolotto V, Djellabi R, Giordana A, Cerrato G, Di Michele A, Bianchi CL (2022) Photocatalytic behaviour of Ag3PO4, Fe3O4 and Ag3PO4/Fe3O4 heterojunction towards the removal of organic pollutants and Cr (VI) from water: efficiency and light-corrosion deactivation. Inorg Chem Commun 141:109516
Brahma S, Nath B, Basumatary B, Das B, Saikia P, Patir K, Basumatary S (2022) Biodiesel production from mixed oils: a sustainable approach towards industrial biofuel production. Chem Eng J Adv 10:100284
Cagnon B, Py X, Guillot A, Stoeckli F, Chambat G (2009) Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Biores Technol 100(1):292–298
Cai H, Zhang D, Ma X, Ma Z (2022) A novel ZnO/biochar composite catalysts for visible light degradation of metronidazole. Sep Purif Technol 288:120633
Carrott P, Carrott MR (2007) Lignin–from natural adsorbent to activated carbon: a review. Biores Technol 98(12):2301–2312
Caturla F, Molina-Sabio M, Rodriguez-Reinoso F (1991) Preparation of activated carbon by chemical activation with ZnCl2. Carbon 29(7):999–1007
Chandra TC, Mirna M, Sudaryanto Y, Ismadji S (2007) Adsorption of basic dye onto activated carbon prepared from durian shell: studies of adsorption equilibrium and kinetics. Chem Eng J 127(1–3):121–129
Chen W-H, Kuo P-C (2010) A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 35(6):2580–2586
Cho J, Davis JM, Huber GW (2010) The intrinsic kinetics and heats of reactions for cellulose pyrolysis and char formation. Chemsuschem 3(10):1162–1165
Colantoni A, Evic N, Lord R, Retschitzegger S, Proto A, Gallucci F, Monarca D (2016) Characterization of biochars produced from pyrolysis of pelletized agricultural residues. Renew Sustain Energy Rev 64:187–194
Collard F-X, Blin J (2014) A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sustain Energy Rev 38:594–608
Creamer AE, Gao B, Zhang M (2014) Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem Eng J 249:174–179
Cruz GJ, Mondal D, Rimaycuna J, Soukup K, Gómez MM, Solis JL, Lang J (2020) Agrowaste derived biochars impregnated with ZnO for removal of arsenic and lead in water. J Environ Chem Eng 8(3):103800
da Cunha Gonçalves G, Pereira NC, Veit MT (2016) Production of bio-oil and activated carbon from sugarcane bagasse and molasses. Biomass Bioenerg 85:178–186
Dahlan I, Lee KT, Kamaruddin AH, Mohamed AR (2006) Key factor in rice husk ash/CaO sorbent for high flue gas desulfurization activity. Environ Sci Technol 40(19):6032–6037
Dai J, Tian S, Jiang Y, Chang Z, Xie A, Zhang R, Yan Y (2018) Facile synthesis of porous carbon sheets from potassium acetate via in-situ template and self-activation for highly efficient chloramphenicol removal. J Alloy Compd 732:222–232
Damma D, Ettireddy PR, Reddy BM, Smirniotis PG (2019) A review of low temperature NH3-SCR for removal of NOx. Catalysts 9(4):349
Danish M, Ahmad T (2018) A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew Sustain Energy Rev 87:1–21
De Macedo LA, Commandre J-M, Rousset P, Valette J, Pétrissans M (2018) Influence of potassium carbonate addition on the condensable species released during wood torrefaction. Fuel Process Technol 169:248–257
Demir-Cakan R, Baccile N, Antonietti M, Titirici M-M (2009) Carboxylate-rich carbonaceous materials via one-step hydrothermal carbonization of glucose in the presence of acrylic acid. Chem Mater 21(3):484–490
Devinny JS, Deshusses MA, Webster TS (2017) Biofiltration for air pollution control. CRC Press
Dhaouadi F, Sellaoui L, Taamalli S, Louis F, El Bakali A, Badawi M, Georgin J, Franco DS, Silva LF, Bonilla-Petriciolet A (2022) Enhanced adsorption of ketoprofen and 2, 4-dichlorophenoxyactic acid on Physalis peruviana fruit residue functionalized with H2SO4: adsorption properties and statistical physics modeling. Chem Eng J 445:136773
Djellabi R, Aboagye D, Galloni MG, Andhalkar VV, Nouacer S, Nabgan W, Rtimi S, Constantí M, Cabello FM, Contreras S (2022) Combined conversion of lignocellulosic biomass into high-value products with ultrasonic cavitation and photocatalytic produced reactive oxygen species-a review. Bioresour Technol 368:128333
Djellabi R, Ali J, Yang B, Haider MR, Su P, Bianchi CL, Zhao X (2020a) Synthesis of magnetic recoverable electron-rich TCTA@ PVP based conjugated polymer for photocatalytic water remediation and disinfection. Sep Purif Technol 250:116954
Djellabi R, Bianchi CL, Haider MR, Ali J, Falletta E, Ordonez MF, Bruni A, Sartirana M, Geioushy R (2021a) Photoactive polymer for wastewater treatment. CRC Press, Nanomaterials for Water Treatment and Remediation, pp 217–244
Djellabi R, Fouzi Ghorab M, Smara A, Bianchi CL, Cerrato G, Zhao X, Yang B (2020b) Titania-Montmorillonite for the photocatalytic removal of contaminants from water: adsorb & shuttle process. Springer, Green materials for wastewater treatment, pp 291–319
Djellabi R, Ghorab FM, Nouacer S, Smara A, Khireddine O (2016) Cr (VI) photocatalytic reduction under sunlight followed by Cr (III) extraction from TiO2 surface. Mater Lett 176:106–109
Djellabi R, Ghorab M (2015) Photoreduction of toxic chromium using TiO2-immobilized under natural sunlight: effects of some hole scavengers and process parameters. Desalin Water Treat 55(7):1900–1907
Djellabi R, Giannantonio R, Falletta E, Bianchi CL (2021b) SWOT analysis of photocatalytic materials towards large scale environmental remediation. Curr Opin Chem Eng 33:100696
Djellabi R, Yang B, Sharif HMA, Zhang J, Ali J, Zhao X (2019a) Sustainable and easy recoverable magnetic TiO2-lignocellulosic biomass@Fe3O4 for solar photocatalytic water remediation. J Clean Prod 233:841–847
Djellabi R, Yang B, Wang Y, Cui X, Zhao X (2019b) Carbonaceous biomass-titania composites with TiOC bonding bridge for efficient photocatalytic reduction of Cr (VI) under narrow visible light. Chem Eng J 366:172–180
Djellabi R, Yang B, Xiao K, Gong Y, Cao D, Sharif HMA, Zhao X, Zhu C, Zhang J (2019c) Unravelling the mechanistic role of TiOC bonding bridge at titania/lignocellulosic biomass interface for Cr (VI) photoreduction under visible light. J Colloid Interface Sci 553:409–417
Djellabi R, Zhang L, Yang B, Haider MR, Zhao X (2019d) Sustainable self-floating lignocellulosic biomass-TiO2@ Aerogel for outdoor solar photocatalytic Cr (VI) reduction. Sep Purif Technol 229:115830
Djellabi R, Zhao X, Bianchi CL, Su P, Ali J, Yang B (2020c) Visible light responsive photoactive polymer supported on carbonaceous biomass for photocatalytic water remediation. J Clean Prod 269:122286
Djellabi R, Zhao X, Ordonez MF, Falletta E, Bianchi CL (2021c) Comparison of the photoactivity of several semiconductor oxides in floating aerogel and suspension systems towards the reduction of Cr (VI) under visible light. Chemosphere 281:130839
Duan D, Feng Z, Dong X, Chen X, Zhang Y, Wan K, Wang Y, Wang Q, Xiao G, Liu H (2021) Improving bio-oil quality from low-density polyethylene pyrolysis: effects of varying activation and pyrolysis parameters. Energy 232:121090
Egirani D, Latif MT, Wessey N, Poyi N, Shehata N (2021) Preparation and characterization of powdered and granular activated carbon from Palmae biomass for mercury removal. Appl Water Sci 11(1):1–11
El Hadrami A, Ojala S, Brahmi R (2022) Production of activated carbon with tunable porosity and surface chemistry via chemical activation of hydrochar with phosphoric acid under oxidizing atmosphere. Surf Interfaces 30:101849
Espinosa-Iglesias D, Valverde-Sarmiento C, Pérez-Cadenas AF, Bautista-Toledo MI, Maldonado-Hódar FJ, Carrasco-Marín F (2015) Mesoporous carbon-xerogels films obtained by microwave assisted carbonization. Mater Lett 141:135–137
Fathy NA (2017) Carbon nanotubes synthesis using carbonization of pretreated rice straw through chemical vapor deposition of camphor. RSC Adv 7(45):28535–28541
Fazal T, Razzaq A, Javed F, Hafeez A, Rashid N, Amjad US, Rehman MSU, Faisal A, Rehman F (2020) Integrating adsorption and photocatalysis: a cost effective strategy for textile wastewater treatment using hybrid biochar-TiO2 composite. J Hazard Mater 390:121623
Fellah I, Djellabi R, Amor HB, Abderrahim N, Bianchi CL, Giordana A, Cerrato G, Di Michele A, Hamdi N (2022) Visible light responsive heterostructure HTDMA-BiPO4 modified clays for effective diclofenac sodium oxidation: role of interface interactions and basal spacing. J Water Process Eng 48:102788
Foo K, Hameed B (2011) Preparation and characterization of activated carbon from sunflower seed oil residue via microwave assisted K2CO3 activation. Biores Technol 102(20):9794–9799
Friehs E, AlSalka Y, Jonczyk R, Lavrentieva A, Jochums A, Walter J-G, Stahl F, Scheper T, Bahnemann D (2016) Toxicity, phototoxicity and biocidal activity of nanoparticles employed in photocatalysis. J Photochem Photobiol, C 29:1–28
Fuertes A, Ferrero G, Sevilla M (2014) One-pot synthesis of microporous carbons highly enriched in nitrogen and their electrochemical performance. J Mater Chem A 2(35):14439–14448
Galloni MG, Bortolotto V, Falletta E, Bianchi CL (2022a) pH-driven selective adsorption of multi-dyes solutions by loofah sponge and polyaniline-modified loofah sponge. Polymers 14(22):4897
Galloni MG, Ferrara E, Falletta E, Bianchi CL (2022b) Olive mill wastewater remediation: from conventional approaches to photocatalytic processes by easily recoverable materials. Catalysts 12(8):923
Gao Y, Yue Q, Gao B, Li A (2020) Insight into activated carbon from different kinds of chemical activating agents: a review. Sci Total Environ 746:141094
Gao Y, Yue Q, Xu S, Gao B (2015) Activated carbons with well-developed mesoporosity prepared by activation with different alkali salts. Mater Lett 146:34–36
García-García A, Chinchón-Yepes S, Linares-Solano A, Salinas-Martínez de Lecea C (1997) NO reduction by potassium-containing coal briquettes. Effect of mineral matter content and coal rank. Energy Fuels 11(2):292–298
Garg D, Kumar S, Sharma K, Majumder C (2019) Application of waste peanut shells to form activated carbon and its utilization for the removal of Acid Yellow 36 from wastewater. Groundw Sustain Dev 8:512–519
Giraldo L, Moreno-Piraján JC (2012) Synthesis of activated carbon mesoporous from coffee waste and its application in adsorption zinc and mercury ions from aqueous solution. E-J Chem 9(2):938–948
Gonçalves MG, da Silva Veiga PA, Fornari MR, Peralta-Zamora P, Mangrich AS, Silvestri S (2020) Relationship of the physicochemical properties of novel ZnO/biochar composites to their efficiencies in the degradation of sulfamethoxazole and methyl orange. Sci Total Environ 748:141381
Gong C, Xue Z, Wen S, Ye Y, Xie X (2016) Advanced carbon materials/olivine LiFePO4 composites cathode for lithium ion batteries. J Power Sources 318:93–112
González-García P (2018) Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew Sustain Energy Rev 82:1393–1414
Gratuito M, Panyathanmaporn T, Chumnanklang R-A, Sirinuntawittaya N, Dutta A (2008) Production of activated carbon from coconut shell: optimization using response surface methodology. Biores Technol 99(11):4887–4895
Guérin T, Ghinet A, Hossart M, Waterlot C (2020) Wheat and ryegrass biomass ashes as effective sorbents for metallic and organic pollutants from contaminated water in lab-engineered cartridge filtration system. Biores Technol 318:124044
Guo F, Wu R, Baxter LL, Hecker WC (2019) Models to predict kinetics of no x reduction by chars as a function of coal rank. Energy Fuels 33(6):5498–5504
Hamada HM, Thomas BS, Tayeh B, Yahaya FM, Muthusamy K, Yang J (2020) Use of oil palm shell as an aggregate in cement concrete: a review. Constr Build Mater 265:120357
Hejazifar M, Azizian S, Sarikhani H, Li Q, Zhao D (2011) Microwave assisted preparation of efficient activated carbon from grapevine rhytidome for the removal of methyl violet from aqueous solution. J Anal Appl Pyrol 92(1):258–266
Heo Y-J, Park S-J (2015) Synthesis of activated carbon derived from rice husks for improving hydrogen storage capacity. J Ind Eng Chem 31:330–334
Hoang AT, Kumar S, Lichtfouse E, Cheng CK, Varma RS, Senthilkumar N, Nguyen PQP, Nguyen XP (2022) Remediation of heavy metal polluted waters using activated carbon from lignocellulosic biomass: an update of recent trends. Chemosphere 302:134825
Hoekman SK, Broch A, Robbins C (2011) Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 25(4):1802–1810
How GTS, Pandikumar A, Ming HN, Ngee LH (2014) Highly exposed 001 facets of titanium dioxide modified with reduced graphene oxide for dopamine sensing. Sci Rep 4(1):1–8
Huang W, Chen W, Fu L, Zhang Y, Wu N, Zhu J, Xu X, Lyu A (2021) Effect analysis of pore wall thickness, pore size, and functional group of activated carbon on adsorption behavior based on molecular simulation. Environ Sci Pollut Res 28(42):59908–59924
Hubetska TS, Kobylinska NG, García JR (2021) Sunflower biomass power plant by-products: properties and its potential for water purification of organic pollutants. J Anal Appl Pyrol 157:105237
Ikram M, Shahid H, Haider J, Haider A, Naz S, Ul-Hamid A, Shahzadi I, Naz M, Nabgan W, Ali S (2022) Nb/starch-doped ZnO nanostructures for polluted water treatment and antimicrobial applications: molecular docking analysis. ACS Omega
Illán-Gómez M, Raymundo-Pinero E, Garcıa-Garcıa A, Linares-Solano A, de Lecea CS-M (1999) Catalytic NOx reduction by carbon supporting metals. Appl Catal B 20(4):267–275
Illán-Gómez M, Salinas-Martínez de Lecea C, Linares-Solano A, Radovic L (1998) Potassium-containing coal chars as catalysts for NOx reduction in the presence of oxygen. Energy Fuels 12(6):1256–1264
Illingworth JM, Rand B, Williams PT (2019) Non-woven fabric activated carbon produced from fibrous waste biomass for sulphur dioxide control. Process Saf Environ Prot 122:209–220
Ioannidou O, Zabaniotou A (2007) Agricultural residues as precursors for activated carbon production—a review. Renew Sustain Energy Rev 11(9):1966–2005
Islam MA, Ahmed M, Khanday W, Asif M, Hameed B (2017) Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol Environ Saf 138:279–285
Jain A, Balasubramanian R, Srinivasan M (2016) Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chem Eng J 283:789–805
Jawad AH, Abdulhameed AS, Wilson LD, Syed-Hassan SSA, ALOthman ZA, Khan MR (2021) High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: optimization and mechanism study. Chinese J Chem Eng 32:281–290
Jeon P, Lee M-E, Baek K (2017) Adsorption and photocatalytic activity of biochar with graphitic carbon nitride (g-C3N4). J Taiwan Inst Chem Eng 77:244–249
Jjagwe J, Olupot PW, Menya E, Kalibbala HM (2021) Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A review. J Bioresour Bioprod 6(4):292–322
Jørgensen S, Pedersen LJT, Jørgensen S, Pedersen LJT (2018) The circular rather than the linear economy. RESTART sustainable business model innovation 103–120
Kalderis D, Bethanis S, Paraskeva P, Diamadopoulos E (2008) Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times. Biores Technol 99(15):6809–6816
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sustain Energy Rev 45:359–378
Kang L, Han L, Wang P, Feng C, Zhang J, Yan T, Deng J, Shi L, Zhang D (2020) SO2-Tolerant NO x reduction by marvelously suppressing SO2 adsorption over FeδCe1− δVO4 catalysts. Environ Sci Technol 54(21):14066–14075
Khaled B, Nassira Z, Imene H (2020) Eco-friendly synthesis of self-regenerative low-cost biosorbent by the incorporation of CuO: a photocatalyst sensitive to visible light irradiation for azo dye removal. Environ Sci Pollut Res 27(25):31074–31091
Khalil H, Jawaid M, Firoozian P, Rashid U, Islam A, Akil HM (2013) Activated carbon from various agricultural wastes by chemical activation with KOH: preparation and characterization. J Biobased Mater Bioenergy 7(6):708–714
Khataee A, Kayan B, Gholami P, Kalderis D, Akay S (2017) Sonocatalytic degradation of an anthraquinone dye using TiO2-biochar nanocomposite. Ultrason Sonochem 39:120–128
Khelaifia FZ, Hazourli S, Nouacer S, Rahima H, Ziati M (2016) Valorization of raw biomaterial waste-date stones-for Cr (VI) adsorption in aqueous solution: thermodynamics, kinetics and regeneration studies. Int Biodeterior Biodegradation 114:76–86
Kim JR, Kan E (2016) Heterogeneous photocatalytic degradation of sulfamethoxazole in water using a biochar-supported TiO2 photocatalyst. J Environ Manage 180:94–101
Komarov VV (2012) Handbook of dielectric and thermal properties of materials at microwave frequencies. Artech House
Koppejan J, Sokhansanj S, Melin S, Madrali S (2012) IEA bioenergy task report-status overview of torrefaction technologies. Procede Biomass: Enschede, The Netherlands 32:1–54
Kordylewski W, Zacharczuk W, Hardy T, Kaczmarczyk J (2005) The effect of the calcium in lignite on its effectiveness as a reburn fuel. Fuel 84(9):1110–1115
Köseoğlu E, Akmil-Başar C (2015) Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv Powder Technol 26(3):811–818
Kosheleva RI, Mitropoulos AC, Kyzas GZ (2019) Synthesis of activated carbon from food waste. Environ Chem Lett 17(1):429–438
Krysanova K, Krylova A, Zaichenko V (2019) Properties of biochar obtained by hydrothermal carbonization and torrefaction of peat. Fuel 256:115929
Kumar A, Kumar A, Sharma G, Ala’a H, Naushad M, Ghfar AA, Guo C, Stadler FJ (2018) Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem Eng J 339:393–410
Kumar A, Kumar A, Sharma G, Naushad M, Stadler FJ, Ghfar AA, Dhiman P, Saini RV (2017) Sustainable nano-hybrids of magnetic biochar supported g-C3N4/FeVO4 for solar powered degradation of noxious pollutants-synergism of adsorption, photocatalysis & photo-ozonation. J Clean Prod 165:431–451
Laird DA, Brown RC, Amonette JE, Lehmann J (2009) Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod Biorefin 3(5):547–562
Lee S-Y, Park S-J (2015) A review on solid adsorbents for carbon dioxide capture. J Ind Eng Chem 23:1–11
Leichtweis J, Silvestri S, Carissimi E (2020) New composite of pecan nutshells biochar-ZnO for sequential removal of acid red 97 by adsorption and photocatalysis. Biomass Bioenerg 140:105648
Li D, Su R, Ma X, Zeng Z, Li L, Wang H (2022a) Porous carbon for oxygenated and aromatic VOCs adsorption by molecular simulation and experimental study: effect pore structure and functional groups. Appl Surf Sci 605:154708
Li H, Dong X, da Silva EB, de Oliveira LM, Chen Y, Ma LQ (2017) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466–478
Li J, Dai J, Liu G, Zhang H, Gao Z, Fu J, He Y, Huang Y (2016a) Biochar from microwave pyrolysis of biomass: a review. Biomass Bioenerg 94:228–244
Li J, Lv F, Yang R, Zhang L, Tao W, Liu G, Gao H, Guan Y (2022b) N-doped biochar from lignocellulosic biomass for preparation of adsorbent: characterization, kinetics and application. Polymers 14(18):3889
Li L, Liu S, Liu J (2011) Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J Hazard Mater 192(2):683–690
Li M, Huang H, Yu S, Tian N, Dong F, Du X, Zhang Y (2016b) Simultaneously promoting charge separation and photoabsorption of BiOX (X= Cl, Br) for efficient visible-light photocatalysis and photosensitization by compositing low-cost biochar. Appl Surf Sci 386:285–295
Li W, Yang K, Peng J, Zhang L, Guo S, Xia H (2008) Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind Crops Prod 28(2):190–198
Li Y-Q, Samad YA, Polychronopoulou K, Alhassan SM, Liao K (2014) Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain Chem Eng 2(6):1492–1497
Li Z, Li Y, Zhu J (2021) Straw-based activated carbon: optimization of the preparation procedure and performance of volatile organic compounds adsorption. Materials 14(12):3284
Ligotski R, Sager U, Schneiderwind U, Asbach C, Schmidt F (2019) Prediction of VOC adsorption performance for estimation of service life of activated carbon based filter media for indoor air purification. Build Environ 149:146–156
Lisowski P, Colmenares JC, Mašek O, Lisowski W, Lisovytskiy D, Grzonka J, Kurzydłowski K (2018) Design and fabrication of TiO2/lignocellulosic carbon materials: relevance of low-temperature sonocrystallization to photocatalysts performance. Chem Cat Chem 10(16):3469–3480
Liu B, Liu Y, Chen H, Yang M, Li H (2017) Oxygen and nitrogen co-doped porous carbon nanosheets derived from Perilla frutescens for high volumetric performance supercapacitors. J Power Sources 341:309–317
Liu D, Quan X, Zhou L, Huang Q, Wang C (2021) Utilization of waste concrete powder with different particle size as absorbents for SO2 reduction. Constr Build Mater 266:121005
Liu H, Li A, Liu Z, Tao Q, Li J, Peng J, Liu Y (2022) Preparation of lightweight and hydrophobic natural biomass-based carbon aerogels for adsorption oils and organic solvents. J Porous Mater 29(4):1001–1009
Liu H, Yu Y, Shao Q, Long C (2019a) Porous polymeric resin for adsorbing low concentration of VOCs: unveiling adsorption mechanism and effect of VOCs’ molecular properties. Sep Purif Technol 228:115755
Liu H, Zhang J, Bao N, Cheng C, Ren L, Zhang C (2012) Textural properties and surface chemistry of lotus stalk-derived activated carbons prepared using different phosphorus oxyacids: adsorption of trimethoprim. J Hazard Mater 235:367–375
Liu Q, Li Y, Chen H, Lu J, Yu G, Möslang M, Zhou Y (2020) Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J Hazard Mater 382:121040
Liu W-J, Tian K, He Y-R, Jiang H, Yu H-Q (2014) High-yield harvest of nanofibers/mesoporous carbon composite by pyrolysis of waste biomass and its application for high durability electrochemical energy storage. Environ Sci Technol 48(23):13951–13959
Liu Z, Adewuyi YG, Shi S, Chen H, Li Y, Liu D, Liu Y (2019b) Removal of gaseous Hg0 using novel seaweed biomass-based activated carbon. Chem Eng J 366:41–49
Lu L, Shan R, Shi Y, Wang S, Yuan H (2019) A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 222:391–398
Lu Y, Cai Y, Zhang S, Zhuang L, Hu B, Wang S, Chen J, Wang X (2022) Application of biochar-based photocatalysts for adsorption-(photo) degradation/reduction of environmental contaminants: mechanism, challenges and perspective. Biochar 4(1):1–24
Lua AC, Lau FY, Guo J (2006) Influence of pyrolysis conditions on pore development of oil-palm-shell activated carbons. J Anal Appl Pyrol 76(1–2):96–102
Lucena SM, Gonçalves DV, Mileo PG, Cavalcante CL (2012) Pore wall thickness and interpore influence on adsorption of alkanes in carbons using explicit pore models. Adsorption 18(2):113–119
Lynam JG, Reza MT, Yan W, Vásquez VR, Coronella CJ (2015) Hydrothermal carbonization of various lignocellulosic biomass. Biomass Conv Bioref 5(2):173–181
Ma CR, Wang T, Zhou J (2020) Removal of heavy metals from aqueous solution using carbon-based adsorbents: a review. J Water Process Eng 37:101339
Ma X, Liu B, Che M, Wu Q, Chen R, Su C, Xu X, Zeng Z, Li L (2021) Biomass-based hierarchical porous carbon with ultrahigh surface area for super-efficient adsorption and separation of acetone and methanol. Sep Purif Technol 269:118690
Manyà JJ, González B, Azuara M, Arner G (2018) Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity. Chem Eng J 345:631–639
Mao H, Huang R, Hashisho Z, Wang S, Chen H, Wang H, Zhou D (2016) Adsorption of toluene and acetone vapors on microwave-prepared activated carbon from agricultural residues: isotherms, kinetics, and thermodynamics studies. Res Chem Intermed 42(4):3359–3371
Marinho BA, Cristóvão RO, Djellabi R, Loureiro JM, Boaventura RA, Vilar VJ (2017) Photocatalytic reduction of Cr (VI) over TiO2-coated cellulose acetate monolithic structures using solar light. Appl Catal B 203:18–30
Masoumi S, Borugadda VB, Nanda S, Dalai AK (2021) Hydrochar: a review on its production technologies and applications. Catalysts 11(8):939
Masoumi S, Dalai AK (2020) Optimized production and characterization of highly porous activated carbon from algal-derived hydrochar. J Clean Prod 263:121427
Matos J, García-López E, Palmisano L, García A, Marcì G (2010) Influence of activated carbon in TiO2 and ZnO mediated photo-assisted degradation of 2-propanol in gas–solid regime. Appl Catal B 99(1–2):170–180
Maximoff SN, Mittal R, Kaushik A, Dhau JS (2022) Performance evaluation of activated carbon sorbents for indoor air purification during normal and wildfire events. Chemosphere 304:135314
Mehta D, Mazumdar S, Singh S (2015) Magnetic adsorbents for the treatment of water/wastewater—a review. J Water Process Eng 7:244–265
Meng F, Song M, Chen Y, Wei Y, Song B, Cao Q (2021) Promoting adsorption of organic pollutants via tailoring surface physicochemical properties of biomass-derived carbon-attapulgite. Environ Sci Pollut Res 28(9):11106–11118
Mian MM, Liu G (2018) Recent progress in biochar-supported photocatalysts: synthesis, role of biochar, and applications. RSC Adv 8(26):14237–14248
Monroy-Figueroa J, Mendoza-Castillo D, Bonilla-Petriciolet A, Pérez-Cruz M (2015) Chemical modification of Byrsonima crassifolia with citric acid for the competitive sorption of heavy metals from water. Int J Environ Sci Technol 12(9):2867–2880
Muley PD, Henkel C, Abdollahi KK, Marculescu C, Boldor D (2016) A critical comparison of pyrolysis of cellulose, lignin, and pine sawdust using an induction heating reactor. Energy Convers Manage 117:273–280
Mumme J, Eckervogt L, Pielert J, Diakité M, Rupp F, Kern J (2011) Hydrothermal carbonization of anaerobically digested maize silage. Biores Technol 102(19):9255–9260
Musa KM, Rushdi SA, Hameed K (2019) Synthesis of activated carbon of lote wood and study its physical properties. J Phys Conf Ser 1362(1):012117
Nabgan W, Abdullah TT, Ikram M, Owgi A, Hatta A, Alhassan M, Aziz F, Jalil A, Van Tran T, Djellabi R (2023) Hydrogen and valuable liquid fuel production from the in-situ pyrolysis-catalytic steam reforming reactions of cellulose bio-polymer wastes dissolved in phenol over trimetallic Ni-La-Pd/TiCa nanocatalysts. J Environ Chem Eng 11(2):109311
Nasri N, Zain H, Usman H, Zulkifli A, Zalilah S, Nurul AS, Nur LA (2015) CO2 adsorption-breakthrough study on activated carbon derived from renewable oil palm empty fruit bunch. Aust J Basic Appl Sci 9(26 Special):67–71
Ng KH, Lai SY, Jamaludin NFM, Mohamed AR (2022) A review on dry-based and wet-based catalytic sulphur dioxide (SO2) reduction technologies. J Hazard Mater 423:127061
Noor NM, Othman R, Mubarak N, Abdullah EC (2017) Agricultural biomass-derived magnetic adsorbents: preparation and application for heavy metals removal. J Taiwan Inst Chem Eng 78:168–177
Nor NM, Lau LC, Lee KT, Mohamed AR (2013) Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—a review. J Environ Chem Eng 1(4):658–666
Norouzi S, Heidari M, Alipour V, Rahmanian O, Fazlzadeh M, Mohammadi-Moghadam F, Nourmoradi H, Goudarzi B, Dindarloo K (2018) Preparation, characterization and Cr (VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Biores Technol 258:48–56
Nouacer S, Hazourli S, Despas C, Hébrant M (2015) Sorption of polluting metal ions on a palm tree frond sawdust studied by the means of modified carbon paste electrodes. Talanta 144:318–323
Nunes L, Matias J, Catalão J (2014) A review on torrefied biomass pellets as a sustainable alternative to coal in power generation. Renew Sustain Energy Rev 40:153–160
Ollis DF (2018) Kinetics of photocatalyzed reactions: five lessons learned. Front Chem 6:378
Olu-Owolabi BI, Alabi AH, Diagboya PN, Unuabonah EI, Düring R-A (2017) Adsorptive removal of 2,4,6-trichlorophenol in aqueous solution using calcined kaolinite-biomass composites. J Environ Manage 192:94–99
Olugbade TO, Ojo OT (2020) Biomass torrefaction for the production of high-grade solid biofuels: a review. BioEnergy Res 13(4):999–1015
Omoriyekomwan JE, Tahmasebi A, Dou J, Wang R, Yu J (2021) A review on the recent advances in the production of carbon nanotubes and carbon nanofibers via microwave-assisted pyrolysis of biomass. Fuel Process Technol 214:106686
Omoriyekomwan JE, Tahmasebi A, Zhang J, Yu J (2019) Mechanistic study on direct synthesis of carbon nanotubes from cellulose by means of microwave pyrolysis. Energy Convers Manage 192:88–99
Ouyang J, Zhou L, Liu Z, Heng JY, Chen W (2020) Biomass-derived activated carbons for the removal of pharmaceutical mircopollutants from wastewater: a review. Sep Purif Technol 253:117536
Ovais M, You M, Ahmad J, Djellabi R, Ali A, Akhtar MH, Abbas M, Chen C (2022) Engineering carbon nanotubes for sensitive viral detection. TrAC Trends Anal Chem 153:116659
Pallarés J, González-Cencerrado A, Arauzo I (2018) Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenerg 115:64–73
Paramasivan B (2022) Microwave assisted carbonization and activation of biochar for energy-environment nexus: a review. Chemosphere 286:131631
Pevida C, Plaza MG, Arias B, Fermoso J, Rubiera F, Pis J (2008) Surface modification of activated carbons for CO2 capture. Appl Surf Sci 254(22):7165–7172
Pi L, Jiang R, Zhou W, Zhu H, Xiao W, Wang D, Mao X (2015) g-C3N4 modified biochar as an adsorptive and photocatalytic material for decontamination of aqueous organic pollutants. Appl Surf Sci 358:231–239
Plaza M, Durán I, Rubiera F, Pevida C (2015) CO2 adsorbent pellets produced from pine sawdust: effect of coal tar pitch addition. Appl Energy 144:182–192
Plaza M, González A, Pevida C, Pis J, Rubiera F (2012) Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl Energy 99:272–279
Plaza M, Rubiera F, Pis J, Pevida C (2010) Ammoxidation of carbon materials for CO2 capture. Appl Surf Sci 256(22):6843–6849
Puma GL, Bono A, Krishnaiah D, Collin JG (2008) Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: a review paper. J Hazard Mater 157(2–3):209–219
Qiu B, Shao Q, Shi J, Yang C, Chu H (2022) Application of biochar for the adsorption of organic pollutants from wastewater: modification strategies, mechanisms and challenges. Sep Purif Technol 300:121925
Rahbar-Shamskar K, Rashidi A, Baniyaghoob S, Khodabakhshi S (2022) In-situ catalytic fast pyrolysis of reed as a sustainable method for production of porous carbon as VOCs adsorbents. J Anal Appl Pyrol 164:105520
Raizada P, Singh P, Kumar A, Sharma G, Pare B, Jonnalagadda SB, Thakur P (2014) Solar photocatalytic activity of nano-ZnO supported on activated carbon or brick grain particles: role of adsorption in dye degradation. Appl Catal A 486:159–169
Raso RA, Zeltner M, Stark WJ (2014) Indoor air purification using activated carbon adsorbers: regeneration using catalytic combustion of intermediately stored VOC. Ind Eng Chem Res 53(49):19304–19312
Raymundo-Pinero E, Cazorla-Amorós D, De Lecea CS-M, Linares-Solano A (2000) Factors controling the SO2 removal by porous carbons: relevance of the SO2 oxidation step. Carbon 38(3):335–344
Redondo E, Carretero-González J, Goikolea E, Ségalini J, Mysyk R (2015) Effect of pore texture on performance of activated carbon supercapacitor electrodes derived from olive pits. Electrochim Acta 160:178–184
Reza MS, Yun CS, Afroze S, Radenahmad N, Bakar MSA, Saidur R, Taweekun J, Azad AK (2020) Preparation of activated carbon from biomass and its’ applications in water and gas purification, a review. Arab J Basic Appl Sci 27(1):208–238
Reza MT, Lynam JG, Uddin MH, Coronella CJ (2013) Hydrothermal carbonization: fate of inorganics. Biomass Bioenerg 49:86–94
Rizhikovs J, Zandersons J, Spince B, Dobele G, Jakab E (2012) Preparation of granular activated carbon from hydrothermally treated and pelletized deciduous wood. J Anal Appl Pyrol 93:68–76
Roa K, Oyarce E, Boulett A, ALSamman M, Oyarzún D, Pizarro GDC, Sánchez J (2021) Lignocellulose-based materials and their application in the removal of dyes from water: a review. Sustain Mater Technol 29:e00320
Roegiers J, Denys S (2021) Development of a novel type activated carbon fiber filter for indoor air purification. Chem Eng J 417:128109
Romano G, Rapposelli A, Marrucci L (2019) Improving waste production and recycling through zero-waste strategy and privatization: an empirical investigation. Resour Conserv Recycl 146:256–263
Rousset P, Macedo L, Commandré J-M, Moreira A (2012) Biomass torrefaction under different oxygen concentrations and its effect on the composition of the solid by-product. J Anal Appl Pyrol 96:86–91
Saber AN, Djellabi R, Fellah I, Abderrahim N, Bianchi CL (2021) Synergistic sorption/photo-Fenton removal of typical substituted and parent polycyclic aromatic hydrocarbons from coking wastewater over CuO-Montmorillonite. J Water Process Eng 44:102377
Salamat S, Younesi H, Bahramifar N (2017) Synthesis of magnetic core–shell Fe3O4@TiO2 nanoparticles from electric arc furnace dust for photocatalytic degradation of steel mill wastewater. RSC Adv 7(31):19391–19405
Sayğılı H, Güzel F (2016) High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J Clean Prod 113:995–1004
Sayğılı H, Sayğılı GA (2019) Optimized preparation for bimodal porous carbon from lentil processing waste by microwave-assisted K2CO3 activation: spectroscopic characterization and dye decolorization activity. J Clean Prod 226:968–976
Seredych M, Bashkova S, Pietrzak R, Bandosz TJ (2010) Interactions of NO2 and NO with carbonaceous adsorbents containing silver nanoparticles. Langmuir 26(12):9457–9464
Sevilla M, Fuertes A (2010) Graphitic carbon nanostructures from cellulose. Chem Phys Lett 490(1–3):63–68
Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrol 89(2):143–151
Shankar Tumuluru J, Sokhansanj S, Hess JR, Wright CT, Boardman RD (2011) A review on biomass torrefaction process and product properties for energy applications. Ind Biotechnol 7(5):384–401
Shen F, Liu J, Zhang Z, Dong Y, Yang Y, Wu D (2018) Oxygen-rich porous carbon derived from biomass for mercury removal: an experimental and theoretical study. Langmuir 34(40):12049–12057
Shen X, Ou R, Lu Y, Yuan A, Liu J, Hu X, Yang Z, Yang F (2020) Engineering adsorption case for efficient capture of VOCs using biomass-based corncobs via a carbonized strategy. Chem Select 5(29):9162–9169
Shen Z, Zhang Y, McMillan O, Jin F, Al-Tabbaa A (2017) Characteristics and mechanisms of nickel adsorption on biochars produced from wheat straw pellets and rice husk. Environ Sci Pollut Res 24(14):12809–12819
Shi K, Yan J, Lester E, Wu T (2014) Catalyst-free synthesis of multiwalled carbon nanotubes via microwave-induced processing of biomass. Ind Eng Chem Res 53(39):15012–15019
Shin J, Lee S-H, Kim S, Ochir D, Park Y, Kim J, Lee Y-G, Chon K (2021) Effects of physicochemical properties of biochar derived from spent coffee grounds and commercial activated carbon on adsorption behavior and mechanisms of strontium ions (Sr2+). Environ Sci Pollut Res 28(30):40623–40632
Shu Y, Zhang F, Wang F, Wang H (2018) Catalytic reduction of NOx by biomass-derived activated carbon supported metals. Chin J Chem Eng 26(10):2077–2083
Shukla P, Giri BS, Mishra RK, Pandey A, Chaturvedi P (2021) Lignocellulosic biomass-based engineered biochar composites: a facile strategy for abatement of emerging pollutants and utilization in industrial applications. Renew Sustain Energy Rev 152:111643
Sirajudheen P, Karthikeyan P, Ramkumar K, Nisheetha P, Meenakshi S (2021) Magnetic carbon-biomass from the seeds of Moringa oleifera@ MnFe2O4 composite as an effective and recyclable adsorbent for the removal of organic pollutants from water. J Mol Liq 327:114829
Skodras G, Diamantopoulou I, Zabaniotou A, Stavropoulos G, Sakellaropoulos G (2007) Enhanced mercury adsorption in activated carbons from biomass materials and waste tires. Fuel Process Technol 88(8):749–758
Šoštarić T, Petrović M, Stojanović J, Marković M, Avdalović J, Hosseini-Bandegharaei A, Lopičić Z (2020) Structural changes of waste biomass induced by alkaline treatment: the effect on crystallinity and thermal properties. Biomass Convers Biorefin 12:2377–2387
Sumathi S, Bhatia S, Lee K, Mohamed A (2009) Performance of an activated carbon made from waste palm shell in simultaneous adsorption of SOx and NOx of flue gas at low temperature. Sci China Ser e: Technol Sci 52(1):198–203
Sumathi S, Bhatia S, Lee K, Mohamed A (2010) SO2 and NO simultaneous removal from simulated flue gas over cerium-supported palm shell activated at lower temperatures− role of cerium on NO removal. Energy Fuels 24(1):427–431
Sun H, Yang B, Li A (2019) Biomass derived porous carbon for efficient capture of carbon dioxide, organic contaminants and volatile iodine with exceptionally high uptake. Chem Eng J 372:65–73
Sun J, Lin X, Xie J, Zhang Y, Wang Q, Ying Z (2020) Facile synthesis of novel ternary g-C3N4/ferrite/biochar hybrid photocatalyst for efficient degradation of methylene blue under visible-light irradiation. Colloids Surf, A 606:125556
Sun Y, Zwolińska E, Chmielewski AG (2016) Abatement technologies for high concentrations of NOx and SO2 removal from exhaust gases: a review. Crit Rev Environ Sci Technol 46(2):119–142
Tamjidi S, Esmaeili H, Moghadas BK (2019) Application of magnetic adsorbents for removal of heavy metals from wastewater: a review study. Mater Res Express 6(10):102004
Tay T, Ucar S, Karagöz S (2009) Preparation and characterization of activated carbon from waste biomass. J Hazard Mater 165(1–3):481–485
Teo HL, Wahab RA (2020) Towards an eco-friendly deconstruction of agro-industrial biomass and preparation of renewable cellulose nanomaterials: a review. Int J Biol Macromol 161:1414–1430
Thompson KA, Shimabuku KK, Kearns JP, Knappe DR, Summers RS, Cook SM (2016) Environmental comparison of biochar and activated carbon for tertiary wastewater treatment. Environ Sci Technol 50(20):11253–11262
To M-H, Hadi P, Hui C-W, Lin CSK, McKay G (2017) Mechanistic study of atenolol, acebutolol and carbamazepine adsorption on waste biomass derived activated carbon. J Mol Liq 241:386–398
Tsai W-T, Chang C, Lee S (1998) A low cost adsorbent from agricultural waste corn cob by zinc chloride activation. Biores Technol 64(3):211–217
Turner A (2018) Black plastics: linear and circular economies, hazardous additives and marine pollution. Environ Int 117:308–318
Wang C, Luo H, Zhang Z, Wu Y, Zhang J, Chen S (2014) Removal of As (III) and As (V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J Hazard Mater 268:124–131
Wang H, Nie L, Li J, Wang Y, Wang G, Wang J, Hao Z (2013) Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin Sci Bull 58(7):724–730
Wang H, Shan L, Lv Q, Cai S, Quan G, Yan J (2020a) Production of hierarchically porous carbon from natural biomass waste for efficient organic contaminants adsorption. J Clean Prod 263:121352
Wang L, Sha L, Zhang S, Cao F, Ren X, Levendis YA (2022) Preparation of activated coke by carbonization, activation, ammonization and thermal treatment of sewage sludge and waste biomass for SO2 absorption applications. Fuel Process Technol 231:107233
Wang L, Zhang Q, Chen B, Bu Y, Chen Y, Ma J, Rosario-Ortiz FL, Zhu R (2020b) Some issues limiting photo (cata) lysis application in water pollutant control: a critical review from chemistry perspectives. Water Res 174:115605
Wang M, Tsai H-S, Zhang C, Wang C, Ho S-H (2021) Effective purification of oily wastewater using lignocellulosic biomass: a review. Chinese Chem Lett 33(6):2807–2816
Wang R, Wang P, Yan X, Lang J, Peng C, Xue Q (2012) Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance. ACS Appl Mater Interfaces 4(11):5800–5806
Wang S, Nam H, Gebreegziabher TB, Nam H (2020c) Adsorption of acetic acid and hydrogen sulfide using NaOH impregnated activated carbon for indoor air purification. Eng Rep 2(1):e12083
Wang T, Liu S, Mao W, Bai Y, Chiang K, Shah K, Paz-Ferreiro J (2020d) Novel Bi2WO6 loaded N-biochar composites with enhanced photocatalytic degradation of rhodamine B and Cr (VI). J Hazard Mater 389:121827
Wang T, Meng D, Zhu J, Chen X (2020e) Effects of pelletizing conditions on the structure of rice straw-pellet pyrolysis char. Fuel 264:116909
Wang T, Zheng L, Liu Y, Tang W, Fang T, Xing B (2020f) A novel ternary magnetic Fe3O4/g-C3N4/carbon layer composite for efficient removal of Cr (VI): a combined approach using both batch experiments and theoretical calculation. Sci Total Environ 730:138928
Wang Y, Lu F, Liu Y, Lu P (2010) Study on NOx reduction and its heterogeneous mechanism during biomass reburning. Proc CSEE 30(26):101–106
Wang Y, Song T, Zhang P, Huang T, Wang T, Wang T, Zeng H (2018) Gas-exfoliation assisted fabrication of porous graphene nanosheets derived from plumeria rubra for highly efficient photocatalytic hydrogen evolution. ACS Sustain Chem Eng 6(9):11536–11546
Wang Z, Lim CJ, Grace JR, Li H, Parise MR (2017) Effects of temperature and particle size on biomass torrefaction in a slot-rectangular spouted bed reactor. Biores Technol 244:281–288
Wong S, Ngadi N, Inuwa IM, Hassan O (2018) Recent advances in applications of activated carbon from biowaste for wastewater treatment: a short review. J Clean Prod 175:361–375
Xiao L-P, Shi Z-J, Xu F, Sun R-C (2012) Hydrothermal carbonization of lignocellulosic biomass. Biores Technol 118:619–623
Xu X, Huang D, Zhao L, Kan Y, Cao X (2016) Role of inherent inorganic constituents in SO2 sorption ability of biochars derived from three biomass wastes. Environ Sci Technol 50(23):12957–12965
Xu X, Tu R, Sun Y, Wu Y, Jiang E, Zhen J (2019) The influence of combined pretreatment with surfactant/ultrasonic and hydrothermal carbonization on fuel properties, pyrolysis and combustion behavior of corn stalk. Biores Technol 271:427–438
Xue K-H, Wang J, Yan Y, Peng Y, Wang W-L, Xiao H-B, Wang C-C (2021) Enhanced As (III) transformation and removal with biochar/SnS2/phosphotungstic acid composites: synergic effect of overcoming the electronic inertness of biochar and W2O3 (AsO4) 2 (As (V)-POMs) coprecipitation. J Hazard Mater 408:124961
Yahya MA, Al-Qodah Z, Ngah CZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sustain Energy Rev 46:218–235
Yan Y, Wang W, Peng Y, Xue K, Wang J, Xiao H (2021) Heterogeneous NiS/NiSe/3D porous biochar for As removal from water by interface engineering-induced nickel lattice distortion. Sci Total Environ 776:145874
Yang F, Li W, Ou R, Lu Y, Dong X, Tu W, Zhu W, Wang X, Li L, Yuan A (2022) Superb VOCs capture engineering carbon adsorbent derived from shaddock peel owning uncompromising thermal-stability and adsorption property. Chin J Chem Eng 47:120–133
Yang K, Peng J, Srinivasakannan C, Zhang L, Xia H, Duan X (2010) Preparation of high surface area activated carbon from coconut shells using microwave heating. Biores Technol 101(15):6163–6169
Yang T, Lua AC (2003) Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J Colloid Interface Sci 267(2):408–417
Yang X, Wan Y, Zheng Y, He F, Yu Z, Huang J, Wang H, Ok YS, Jiang Y, Gao B (2019) Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chem Eng J 366:608–621
Ye S, Yan M, Tan X, Liang J, Zeng G, Wu H, Song B, Zhou C, Yang Y, Wang H (2019) Facile assembled biochar-based nanocomposite with improved graphitization for efficient photocatalytic activity driven by visible light. Appl Catal B 250:78–88
Yin J, Zhang D, Zhao J, Wang X, Zhu H, Wang C (2014) Meso-and micro-porous composite carbons derived from humic acid for supercapacitors. Electrochim Acta 136:504–512
Yorgun S, Yıldız D (2015) Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J Taiwan Inst Chem Eng 53:122–131
Yousaf B, Liu G, Ali MU, Abbas Q, Liu Y, Ullah H, Cheema AI (2021) Decisive role of vacuum-assisted carbonization in valorization of lignin-enriched (Juglans regia-shell) biowaste. Biores Technol 323:124541
Yu F, Tian F, Zou H, Ye Z, Peng C, Huang J, Zheng Y, Zhang Y, Yang Y, Wei X (2021) ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J Hazard Mater 415:125511
Zhang C, Jiang X, Huang X, Liu J (2007) Characteristics of adsorption of NO gas on coal char and FTIR analysis. J Chem Ind Eng-China 58(3):581
Zhang W, Rabiei S, Bagreev A, Zhuang M, Rasouli F (2008) Study of NO adsorption on activated carbons. Appl Catal B 83(1–2):63–71
Zhang X, Li J, Yang W, Blasiak W (2011) Formation mechanism of levoglucosan and formaldehyde during cellulose pyrolysis. Energy Fuels 25(8):3739–3746
Zhang C, Song W, Ma Q, Xie L, Zhang X, Guo H (2016a) Enhancement of CO2 capture on biomass-based carbon from black locust by KOH activation and ammonia modification. Energy Fuels 30(5):4181–4190
Zhang Z, Wang T, Ke L, Zhao X, Ma C (2016b) Powder-activated semicokes prepared from coal fast pyrolysis: influence of oxygen and steam atmosphere on pore structure. Energy Fuels 30(2):896–903
Zhang H, Wang Z, Li R, Guo J, Li Y, Zhu J, Xie X (2017) TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 185:351–360
Zhang J, Li Y, Li L, Li W, Yang C (2018a) Dual functional N-doped TiO2-carbon composite fibers for efficient removal of water pollutants. ACS Sustain Chem Eng 6(10):12893–12905
Zhang Y, Xu M, Li H, Ge H, Bian Z (2018b) The enhanced photoreduction of Cr (VI) to Cr (III) using carbon dots coupled TiO2 mesocrystals. Appl Catal B 226:213–219
Zhang Z, Jiang C, Li D, Lei Y, Yao H, Zhou G, Wang K, Rao Y, Liu W, Xu C (2020b) Micro-mesoporous activated carbon simultaneously possessing large surface area and ultra-high pore volume for efficiently adsorbing various VOCs. Carbon 170:567–579
Zhang X-X, Lü X-X, Wu H-X, Miao X, Lin R-Y, Zhou Z-J (2020a) Microscopic mechanism for effect of sodium on NO heterogeneous reduction by char. J Fuel Chem Technol 48(6):663–673
Zhang J, Shao J, Huang D, Feng Y, Zhang X, Zhang S, Chen H (2020c) Influence of different precursors on the characteristic of nitrogen-enriched biochar and SO2 adsorption properties. Chem Eng J 385:123932
Zhao M, Xue P, Liu J, Liao J, Guo J (2021a) A review of removing SO2 and NOX by wet scrubbing. Sustain Energy Technol Assess 47:101451
Zhao W, Geng X, Lu J, Duan Y, Liu S, Hu P, Xu Y, Huang Y, Tao J, Gu X (2021b) Mercury removal performance of brominated biomass activated carbon injection in simulated and coal-fired flue gas. Fuel 285:119131
Zhao Y, Shi J, Wang X, Li W, Wu Y, Jiang Z (2020) Biomass@ MOF-derived carbon aerogels with a hierarchically structured surface for treating organic pollutants. Ind Eng Chem Res 59(39):17529–17536
Zhong B, Shi W, Fu W (2002) Importance of heterogeneous mechanisms of NO reduction during reburning with pulverized coals. J Combustion Sci Technol 8:6–9
Zhong L-X, Peng X-W, Yang D, Sun R-C (2012) Adsorption of heavy metals by a porous bioadsorbent from lignocellulosic biomass reconstructed in an ionic liquid. J Agric Food Chem 60(22):5621–5628
Zhou S, Zhang C, Xia L, Fu Z, Zhu N, Gong J, Wang X, Lyu P, Li L, Xu W (2022) A flexible and weavable lignocellulose-based photocatalyst supported by natural three-dimensional porous juncus effusus for highly efficient degradation of environmental contaminants. ACS Appl Mater Interfaces 14(24):27955–27967
Zhu J, Jia J, Kwong FL, Ng DHL, Tjong SC (2012) Synthesis of multiwalled carbon nanotubes from bamboo charcoal and the roles of minerals on their growth. Biomass Bioenerg 36:12–19
Zhu J, Li Y, Xu L, Liu Z (2018) Removal of toluene from waste gas by adsorption-desorption process using corncob-based activated carbons as adsorbents. Ecotoxicol Environ Saf 165:115–125
Ziati M, Hazourli S, Nouacer S, Khelaifia FZ (2012) Elimination of arsenic (III) by adsorption on coal resulting from date pits and activated thermally and chemically. Water Qual Res J Can 47(1):91–102
Zieliński M, Wojcieszak R, Monteverdi S, Mercy M, Bettahar M (2005) Hydrogen storage on nickel catalysts supported on amorphous activated carbon. Catal Commun 6(12):777–783
Funding
Open access funding provided by Universitat Rovira i Virgili. Authors are thankful for the support from Grant PID2021-123665OB-I00 and TED2021-129343B-I00 funded by MCIN/AEI/ 10.13039/501100011033 and, as appropriate, by “ERDF A way of making Europe”, by the “European Union” or by the “European Union NextGenerationEU/PRTR”. Dr Ridha Djellabi acknowledges Maria Zambrano Grants-2021URV-MZ-15.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. M. Mergbi: writing—original draft preparation and conceptualization. M. G. Galloni, D. Aboagye. E. Elimian, P. Su, B.M. Ikram: writing and review, W. Nabgan, J. Bedia, H.B. Amor, S. Contreras, F. Medina: revision and editing, R. Djellabi: writing, revision and supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
The study does not involve any human participants, human data, or human tissues.
Consent for publication
All authors have read and approved the final manuscript to be published.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mergbi, M., Galloni, M.G., Aboagye, D. et al. Valorization of lignocellulosic biomass into sustainable materials for adsorption and photocatalytic applications in water and air remediation. Environ Sci Pollut Res 30, 74544–74574 (2023). https://doi.org/10.1007/s11356-023-27484-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27484-2