Abstract
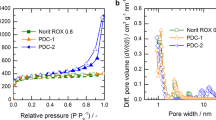
Carbon porous materials obtained through KOH activation of a furfural + hydroquinone + urotropine mixture were applied as adsorbent for the remediation of methylene blue (MB). The impact of porous structure with special attention to pore size distribution along with well-known pore volume and specific surface area on the remediation of MB was well investigated and elucidated. Findings obtained revealed that pore size distribution plays a crucial role in the liquid-phase adsorption of organic dyes like MB. By varying the synthesis mode parameters, in particular, the activating agent/precursor mass ratio, with the composition and initial components ratios remaining unchanged, samples with different pore size distribution were obtained. It was found that the material predominantly containing pores with an average equivalent diameter of ~ 3.5 nm appears to be the efficient MB adsorbent. The resulting highly porous carbon materials demonstrated high MB adsorption capacity (up to 2555 mg/g). Furthermore, to fully elucidate the adsorption mechanisms occurring on the obtained materials, a comprehensive mathematical processing of experimental data was performed out using the known kinetic and diffusion models (pseudo-first- and pseudo-second order, and intraparticle diffusion), as well as adsorption equilibrium isotherm models (Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich).
It can be concluded that the porous carbon materials obtained and described in the present work are effective adsorbents for the removal of MB and may possess great potential for the treatment of dye-containing wastewater.






Similar content being viewed by others
Availability of data and materials
This published article is included in all data generated or analysed during this study.
Abbreviations
- MB:
-
Methylene blue
- PAHs:
-
Poly aromatic hydrocarbons
- CPs:
-
Chlorophenols
- BET:
-
Brunauer–Emmett–Teller
- ACM:
-
Activated carbon material
- DFT:
-
Density functional theory
- PSD:
-
Pore size distribution
References
Asfaram A, Ghaedi M, Agarwal S, Tyagi I, Gupta VK (2015) Removal of basic dye Auramine-O by ZnS: Cu nanoparticles loaded on activated carbon: optimization of parameters using response surface methodology with central composite design. RSC Adv 5(24):18438–18450
Aysan H, Edebali S, Ozdemir C, Karakaya MC, Karakaya N (2016) Use of chabazite, a naturally abundant zeolite, for the investigation of the adsorption kinetics and mechanism of methylene blue dye. Microporous Mesoporous Mater 235:78–86
Bhatt AS, Sakaria PL, Vasudevan M, Pawar RR, Sudheesh N, Bajaj HC, Mody HM (2012) Adsorption of an anionic dye from aqueous medium by organoclays: equilibrium modeling, kinetic and thermodynamic exploration. RSC Adv 2(23):8663–8671
Bhattacharyya KG, Sharma A (2004) Azadirachta indica leaf powder as an effective biosorbent for dyes: a case study with aqueous Congo Red solutions. J Environ Manage 71(3):217–229
Boyd GE, Adamson AW, Myers Jr LS (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics1. J Am Chem Soc 69(11):2836–2848
Bu J, Yuan L, Zhang N, Liu D, Meng Y, Peng X (2020) High-efficiency adsorption of methylene blue dye from wastewater by a thiosemicarbazide functionalized graphene oxide composite. Diam Relat Mater 101:107604
Chang J, Ma J, Ma Q, Zhang D, Qiao N, Hu M, Ma H (2016) Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl Clay Sci 119:132–140
Chen S, Qin C, Wang T, Chen F, Li X, Hou H, Zhou M (2019) Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J Mol Liq 285:62–74
Danish M, Ahmad T, Majeed S, Ahmad M, Ziyang L, Pin Z, Iqubal SS (2018) Use of banana trunk waste as activated carbon in scavenging methylene blue dye: kinetic, thermodynamic, and isotherm studies. Bioresource Technology Reports 3:127–137
Dubinin M (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev 60(2):235–241
Erdogan FO, Kopac T (2019) Adsorption behavior of alcohol vapors on Zonguldak-Karadon coal derived porous carbons. Energy Sour Part A: Recov Util Environ Effects. https://doi.org/10.1080/15567036.2019.1666191
Ezzeddine Z, Batonneau-Gener I, Pouilloux Y, Hamad H (2016) Removal of methylene blue by mesoporous CMK-3: kinetics, isotherms and thermodynamics. J Mol Liq 223:763–770
Gao JJ, Qin YB, Zhou T, Cao DD, Xu P, Hochstetter D, Wang YF (2013) Adsorption of methylene blue onto activated carbon produced from tea (Camellia sinensis L.) seed shells: kinetics, equilibrium, and thermodynamics studies. J Zhejiang Univ Sci B 14(7):650–658
Ghaedi M, Hajjati S, Mahmudi Z, Tyagi I, Agarwal S, Maity A, Gupta VK (2015) Modeling of competitive ultrasonic assisted removal of the dyes–methylene blue and safranin-O using Fe3O4 nanoparticles. Chem Eng J 268:28–37
Giusto LA, Pissetti FL, Castro TS, Magalhães F (2017) Preparation of activated carbon from sugarcane bagasse soot and methylene blue adsorption. Water Air Soil Pollut 228(7):1–10
Guesmi Y, Agougui H, Lafi R, Jabli M, Hafiane A (2018) Synthesis of hydroxyapatite-sodium alginate via a co-precipitation technique for efficient adsorption of methylene blue dye. J Mol Liq 249:912–920
Guo RF, Zhao X, Li XY, Liu ZH (2021) Preparation and formation mechanism of graphene oxide supported hollow mesoporous Mg2Si3O6 (OH) 4 micro-nanospheres with highly efficient methylene blue dye removal from wastewater. Colloids Surf, A 610:125936
Gupta VK, Nayak A, Agarwal S, Chaudhary M, Tyagi I (2014) Removal of Ni (II) ions from water using scrap tire. J Mol Liq 190:215–222
Gupta VK, Moradi O, Tyagi I, Agarwal S, Sadegh H, Shahryari-Ghoshekandi R, Makhlouf ASH, Goodarzi M, Garshasbi A (2016) Study on the removal of heavy metal ions from industry waste by carbon nanotubes: effect of the surface modification: a review. Crit Rev Environ Sci Technol 46(2):93–118
Heidarinejad Z, Rahmanian O, Fazlzadeh M, Heidari M (2018) Enhancement of methylene blue adsorption onto activated carbon prepared from Date Press Cake by low frequency ultrasound. J Mol Liq 264:591–599
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70(2):115–124
Ibupoto AS, Qureshi UA, Ahmed F, Khatri Z, Khatri M, Maqsood M, Brohi RZ, Kim IS (2018) Reusable carbon nanofibers for efficient removal of methylene blue from aqueous solution. Chem Eng Res Des 136:744–752
Ighalo JO, Iwuozor KO, Igwegbe CA, Adeniyi AG (2021) Verification of pore size effect on aqueous-phase adsorption kinetics: a case study of methylene blue. Colloids Surf, A 626:127119
Islam MA, Ahmed MJ, Khanday WA, Asif M, Hameed BH (2017) Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. J Environ Manage 203:237–244
Jawad AH, Abdulhameed AS (2020) Statistical modeling of methylene blue dye adsorption by high surface area mesoporous activated carbon from bamboo chip using KOH-assisted thermal activation. Energ Ecol Environ 5:456–469
Jia P, Tan H, Liu K, Gao W (2018) Removal of methylene blue from aqueous solution by bone char. Appl Sci 8(10):1903
Jung KW, Choi BH, Hwang MJ, Jeong TU, Ahn KH (2016) Fabrication of granular activated carbons derived from spent coffee grounds by entrapment in calcium alginate beads for adsorption of acid orange 7 and methylene blue. Biores Technol 219:185–195
Kannan N, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—a comparative study. Dyes Pigm 51(1):25–40
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Liu J, Li E, You X, Hu C, Huang Q (2016) Adsorption of methylene blue on an agro waste oiltea shell with and without fungal treatment. Sci Rep 6(1):1–10
Liu XJ, Li MF, Singh SK (2021) Manganese-modified lignin biochar as adsorbent for removal of methylene blue. J Market Res 12:1434–1445
Ludwinowicz J, Jaroniec M (2015) Effect of activating agents on the development of microporosity in polymeric-based carbon for CO2 adsorption. Carbon 94:673–679
Ma X, Zhang F, Zhu J, Yu L, Liu X (2014) Preparation of highly developed mesoporous activated carbon fiber from liquefied wood using wood charcoal as additive and its adsorption of methylene blue from solution. Biores Technol 164:1–6
Malash GF, El-Khaiary MI (2010) Piecewise linear regression: a statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem Eng J 163(3):256–263
Marrakchi F, Khanday WA, Asif M, Hameed BH (2016) Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int J Biol Macromol 93:1231–1239
Meili L, Lins PV, Zanta CLPS, Soletti JI, Ribeiro LMO, Dornelas CB, Silva TL, Vieira MGA (2019) MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl Clay Sci 168:11–20
Memetova A, Tyagi I, Karri RR, Memetov N, Zelenin A, Stolyarov R, Babkin A, Yagubov V, Burmistrov I, Tkachev A, Bogoslovskiy V (2022) High-density nanoporous carbon materials as storage material for methane: a value-added solution. Chem Eng J 433:134608
Nandiyanto ABD, Arinalhaq ZF, Rahmadianti S, Dewi MW, Rizky YPC, Maulidina A, Anggraeni S, Bilad MR, Yunas J (2020) Curcumin adsorption on carbon microparticles: synthesis from soursop (annonamuricata l.) peel waste, adsorption isotherms and thermodynamic and adsorption mechanism. Int J Nanoelectr Mater 13 (Special issue):173–192
Nayak A, Bhushan B, Gupta V, Sharma P (2017) Chemically activated carbon from lignocellulosic wastes for heavy metal wastewater remediation: effect of activation conditions. J Colloid Interface Sci 493:228–240
Nayak SS, Mirgane NA, Shivankar VS, Pathade KB, Wadhawa GC (2021) Adsorption of methylene blue dye over activated charcoal from the fruit peel of plant hydnocarpus pentandra. Materials Today: Proceedings 37:2302–2305
Reichenberg D (1953) Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange. J Am Chem Soc 75(3):589–597
Samuel MS, Suman S, Selvarajan E, Mathimani T, Pugazhendhi A (2020) Immobilization of Cu3 (btc) 2 on graphene oxide-chitosan hybrid composite for the adsorption and photocatalytic degradation of methylene blue. J Photochem Photobiol, B 204:111809
Saraf S, Vaidya V (2016) Elucidation of sorption mechanism of R. arrhizus for reactive blue 222 using equilibrium and kinetic studies. J Microb Biochem Technol 8(3):236–46
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619
Soudagar S, Akash S, Venkat MS, Poiba VR, Vangalapati M (2022) Adsorption of methylene blue dye on nano graphene oxide-thermodynamics and kinetic studies. Mater Today: Proc 59:667–672
Spagnoli AA, Giannakoudakis DA, Bashkova S (2017) Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: the role of surface and structural parameters. J Mol Liq 229:465–471
Stavrinou A, Aggelopoulos CA, Tsakiroglou CD (2018) Exploring the adsorption mechanisms of cationic and anionic dyes onto agricultural waste peels of banana, cucumber and potato: adsorption kinetics and equilibrium isotherms as a tool. J Environ Chem Eng 6(6):6958–6970
Tan IAW, Ahmad AL, Hameed BH (2009) Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2, 4, 6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J Hazard Mater 164:473–482
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Wang Y, López-Valdivieso A, Zhang T, Mwamulima T, Zhang X, Song S, Peng C (2017) Preparation of microscale zero-valent iron-fly ash-bentonite composite and evaluation of its adsorption performance of crystal violet and methylene blue dyes. Environ Sci Pollut Res 24(24):20050–20062
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–59
Yusop MFM, Ahmad MA, Rosli NA, Abd Manaf ME (2021) Adsorption of cationic methylene blue dye using microwave-assisted activated carbon derived from acacia wood: optimization and batch studies. Arab J Chem 14(6):103122
Zbair M, Anfar Z, Khallok H, Ahsaine HA, Ezahri M, Elalem N (2018) Adsorption kinetics and surface modeling of aqueous methylene blue onto activated carbonaceous wood sawdust. Fullerenes, Nanotubes, Carbon Nanostruct 26(7):433–442
Zhao X, Bu X, Wu T, Zheng ST, Wang L, Feng P (2013) Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat Commun 4(1):1–9
Zou Y, Wang X, Ai Y, Liu Y, Li J, Ji Y, Wang X (2016) Coagulation behavior of graphene oxide on nanocrystallined Mg/Al layered double hydroxides: batch experimental and theoretical calculation study. Environ Sci Technol 50(7):3658–3667
Funding
The study was supported by project 22–13-20074 of Russian Science Foundation.
Author information
Authors and Affiliations
Contributions
Inderjeet Tyagi, Anastasia Memetova: Conceptualization; project administration; and supervision.
Pratibha Singh, Suhas, Joanna Goscianska, Alexander Burakov, Irina Burakova: Data curation; formal analysis; software; visualization.
Elina Mkrtchyan, Nariman Memetov, Alena Gerasimova, Gulnara Shigabaeva, Evgeny Galunin Ajay Kumar: Investigation; methodology, software, validation; visualization.
All authors: Writing, original draft; writing, review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Memetova, A., Tyagi, I., Suhas et al. Porous material based on modified carbon and the effect of pore size distribution on the adsorption of methylene blue dye from an aqueous solution. Environ Sci Pollut Res 30, 22617–22630 (2023). https://doi.org/10.1007/s11356-022-23486-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23486-8