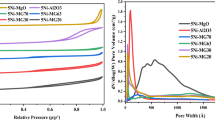
Abstract
In this work, we studied the effect of CO2 in the feed stream of the TRM process performance of nickel supported on LaFeO3 perovskite for hydrogen production compared to the POM reaction. The perovskite and nickel supported on LaFeO3 were synthesized and characterized by thermogravimetric analysis (TGA/DTA), X-ray diffraction (XRD), transmission electron microscopy (TEM), and programmed reduction temperature (TPR). The catalytic tests were carried out in temperatures varying from 700 to 800 °C with feed flow of 350 cm3/min and 200 cm3/min for TRM and POM, respectively. The hydrogen selectivity for the tri-reforming was 78%, while for the partial oxidation reaction, only 55% H2 at 700 °C. Results showed that the hydrogen selectivity for the Ni/LaFeO3 catalyst is significantly higher for the tri-reforming process, suggesting that CO2 enhanced the hydrogen selectivity compared to the partial oxidation of methane. Analyses by Raman spectroscopy and thermogravimetric calculations showed structural modifications of the catalysts after the reaction. The Raman spectrum showed segregated NiO and Fe3O4 and low carbon formation at 700 °C. The proposed mechanism suggests methane and oxygen adsorption, lattice oxygen and CO2 on surface active sites, and vacancies for both reactions.









Similar content being viewed by others
Data availability
Not applicable.
References
Anchieta CG, Assaf EM, Assaf JM (2022) Syngas production by methane tri-reforming: Effect of Ni/CeO2 synthesis method on oxygen vacancies and coke formation, Journal of CO2 Utilization, v56, 101853,ISSN 2212-9820
Balat M (2009) Energ Source Part A 31:39
Dai X, Yu C, Wu Q (2008) Comparison of LaFeO3, La0.8Sr0.2FeO3, and La0.8Sr0.2Fe0.9Co0.1O3 perovskite oxides as oxygen carrier for partial oxidation of methane. J Nat Gas Chem 17:415
Fabrik M, Salama A, Ibrahim H (2021) State-of-the-art in methane-reforming reactor modeling: challenges and new insights. Rev Chem Eng, Vol. (Issue), pp. 000010151520200038. https://doi.org/10.1515/revce-2020-0038
Farhadi S, Momeni Z, Taherimehr M (2009) Rapid synthesis of perovskite-type LaFeO3 nanoparticles by microwave-assisted decomposition of bimetallic La[Fe(CN)6].5H2O compound. J Alloys Compd 471:5–8
Fedorova ZA, Danilova MM, Zaikovskii VI (2020) Porous nickel-based catalysts for tri-reforming of methane to synthesis gas: catalytic activity. Mater Lett 261:Article 127087
Galasso FS (1969) Structure, properties and preparation of perovskites-type compunds: international series of monographs in solid state physics. Pergamon Press, pp 8–10
García-Vargas JM, Valverde JL, Diez J (2015) Catalytic and kinetic analysis of the methane tri-reforming over a Ni–Mg/β-SiC catalyst. Int J Hydrog Energy 40:8677
Guo S, Wang J, Ding C, Duan Q, Ma Q, Zhang K, Liu P (2018) Confining Ni nanoparticles in honeycomb-like silica for coking and sintering resistant partial oxidation of methane. Int J Hydrog Energy 43:6603
Hai NH, Phu ND, Luong NH, Chau N, Chinh HD, Hoang LH, Leslie-Pelecky DL (2008) Mechanism for sustainable magnetic nanoparticles under ambient conditions. J Korean Phys Soc 52:1327
Khan A, Smirniotis PG (2008) Copper promotion of chromium-doped iron oxide water-gas shift catalysts under industrially relevant conditions. J Mol Catal A-Chem 280:43–51
Kumar R, Kumar K, Choudary NV, Pant KK (2019) Effect of support materials on the performance of Ni-based catalysts in tri-reforming of methane. Fuel Process Technol 186:40
Kumar R, Kumar K, Pant KK, Choudary NV (2020) Tuning the metal-support interaction of methane tri-reforming catalysts for industrial flue gas utilization, Int.l J. of Hydrogen Energy, v 45, Issue 3, p 1911-1929,ISSN 0360-3199
Landon J, Demeter E, Inoglu N, Keturakis C, Wachs IE, Vasic R, Frenkel AI, Kitchin JR (2012) Spectroscopic characterization of mixed Fe–Ni oxide electrocatalysts for the oxygen evolution reaction. Alkaline Electrolytes, ACS Catal 2:1793
Lazarević ZŽ, Jovalekić Č, Milutinović A, Sekulić D, Ivanovski VN, Rečnik A, Cekić B, Romčević NŽ (2013) Nanodimensional spinel NiFe2O4 and ZnFe2O4 ferrites prepared by soft mechanochemical synthesis. J Appl Phys 113:187221
Lee W-Y, Yun HJ, Yoon J-W (2014) Characterization and magnetic properties of LaFeO3 nanofibers synthesized by electrospinning. J Alloys Compd 583:320
Melchiori T, Di Felice L, Mota N, Navarro RM, Fierro JLG, van Sint Annaland M, Gallucci F (2014) Methane partial oxidation over a LaCr0.85Ru0.15O3 catalyst: characterization, activity tests and kinetic modeling. Appl Catal A-Gen 486:239. https://doi.org/10.1016/j.apcata.2014.08.040
Minh DP, Siang TJ, Vo DVN, Phan TS, Ridart C, Nzihou A, Grouse D (2018), in: Hydrogen supply chains design, deployment and operation. Academic Press, ch. 4 pp.111-166
Mironova-Ulmane N, Kuzmin A, Steins I, Grabis J, Sildos I, Pärs M (2007) Raman scattering in nanosized nickel oxide NiO. J Phys Conf Ser 93:012039
Pham X, Ashik UPM, Hayashi J, Pérez Alonso A, Pla D, Gómez M, Minh DP (2021) Study on the promotional effect of lanthana addition on the performance of hydroxyapatite-supported Ni catalysts for the CO2 methanation reaction, Appl. Catal. A-Gen., 623, Article 118286
Pino L, Vita A, Cipitì F, Laganà M, Recupero V (2011) Hydrogen production by methane tri-reforming process over Ni–ceria catalysts: Effect of La-doping. Appl Catal B Environ 104:64–73. https://doi.org/10.1016/j.apcatb.2011.02.027
Roseno KTC, Brackmann R, da Silva MA, Schmal M (2016) Investigation of LaCoO3, LaFeO3 and LaCo0.5Fe0.5O3 perovskites as catalyst precursors for syngas production by partial oxidation of methane. Int J Hydrog Energy 41:18178
Rousseau DL, Bauman RP, Porto SPS (1981) J Raman Spectrosc 10:253
Schmal M, Toniolo FS, Kozonoe CE (2018) Perspective of catalysts for (Tri) reforming of natural gas and flue gas rich in CO2. Appl Catal A-Gen 568:23
Socrates G (2004) Infrared and Raman characteristic group frequencies: tables and charts, Third. ed. [S.l.]: John Wiley & Sons
M.A.G. Soler, F. Qu, Raman spectroscopy of iron oxide (2012) In: KUMAR, C.S.S.R. Raman spectroscopy for nanomaterials characterization. Baton Rouge: Springer, 379-416
Wachter P, Gaber C, Demuth M, Hochenauer C (2020) Experimental investigation of tri-reforming on a stationary, recuperative TCR-reformer applied to an oxy-fuel combustion of natural gas, using a Ni-catalyst, Energy, v 212, 118719, ISSN 0360-5442
Zhao K, He F, Huang Z, Zheng A, Li H, Zhao Z (2014) La1-xSrxFeO3 perovskites as oxygen carriers for the partial oxidation of methane to syngas. Chin J Catal 35:1196
Zhao K, Zheng A, Li H, He F, Huang Z, Wei G, Shen Y, Zhao Z (2017) Exploration of the mechanism of chemical looping steam methane reforming using double perovskite-type oxides La1.6Sr0.4FeCoO6. Appl Catal B-Environ 219:672
Funding
The authors acknowledge financial support by CNPq (grant numbers 159749/2015-8 and 309444/2016-0), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES, Finance Code 001), FAPESP (grant number 2017/17283-6), and FAPESP-Shell Brasil—Research Center for Gas Innovation—RCGI (FAPESP grant number 2014/50279-4).
Author information
Authors and Affiliations
Contributions
Auta Soares: conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, and visualization. K. T. C. Roseno: investigation, resources, data curation, writing—review and editing, and visualization. Reinaldo Giudici: validation, investigation, writing—review and editing, and visualization. Martin Schmal: conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization, supervision, and project administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1194 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soares, A.N.d.B., Roseno, K.T.C., Giudici, R. et al. The effect of carbon dioxide in the feed stream of tri-reforming of methane process compared to the partial oxidation of methane. Environ Sci Pollut Res 30, 19111–19119 (2023). https://doi.org/10.1007/s11356-022-23474-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23474-y