Abstract
Thermo-mineral springs are widely spread over the volcanic areas of Salerno, a city in southern Italy. Although the water of thermal structures provides beneficial effects on human health, the air is characterized by the presence of potentially toxic compounds, such as hydrogen sulphide (H2S) and sulphur dioxide (SO2). Exposure to sulphurous compounds may have detrimental effects on human health, with asthma being the most common. In this study, air concentrations of H2S and SO2 in the thermal springs of Contursi Terme (Salerno, Italy) were monitored for 4 months (using both active and passive sampling), along with the chemical and microclimatic characterization of thermal water, to assess workers’ exposure to these pollutants. An in-depth characterization of indoor air at the springs is paramount to establish emission control limits for occupational exposure and to take protective measures. The air concentration of SO2 varied from 0.11 ± 0.02 to 0.91 ± 0.02 mg/m3, following a seasonal pattern (higher values in winter and lower in spring). Conversely, indoor H2S concentrations did not vary significantly with time, but outdoor levels (from 0.40 ± 0.03 to 1.90 ± 0.03 mg/m3) were always higher than indoor ones (from 0.11 ± 0.03 to 0.56 ± 0.03 mg/m3). Not negligible air concentrations of these pollutants were detected in this thermal spring workplace, so further investigations are needed to ensure workers’ safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermal springs are mineral waters with a specific saline composition (Quattrini et al. 2016). These environments have been known as early as the first century A.D. when their presence was reported in ancient literature and the use of thermal baths for curative purposes was well known since Roman times (Routh et al. 1996; Frosch 2007; Valeriani et al. 2018). The thermo-mineral springs received great attention in the culture of bathing and personal hygiene for specific therapeutic uses (Jackson 1990; Croutier 1992; Frost 2004; Torres-Ceron et al. 2019; Costantino et al. 2020). Although the water of thermal structures provides beneficial effects on human health, the atmosphere of these environments is characterized by the presence of sulphurous compounds such as hydrogen sulphide (H2S) and sulphur dioxide (SO2) (Attene-Ramos et al. 2006; Stanhope et al. 2017; Pironti et al. 2021a, b ; Ricciardi et al. 2021). H2S is an undesirable air pollutant because of its malodour and toxicity even at low concentrations (< 10 ppm) (Elwood 2021). Concerns about health effects are mostly related to the brain and central nervous system, with the risk of damage depending on both the length of the exposure and the concentration of H2S (EPA 1986; Legator and Singleton 1997; Lim et al. 2016; Nuvolone et al. 2019).
Air pollutants could interact with the body via skin contact, the respiratory tract, or even oral intake, which pose various health hazards, such as respiratory irritation (Rafieepour et al. 2013) and oral disease (Vianna et al. 2005), and may even increase the risk of cancer (IARC 1992; NTP 2016; Ghantous et al. 2015). Irritants such as acid gases are less known than sensitizers as causative agents of occupational respiratory diseases, which hampers the recognition and the understanding of the hazards of irritative agents at workplaces. The health impact of irritants is potentially high because persistent symptoms and abnormal lung function have still been reported years after diagnosis. Recently, the role of irritants in pulmonary disease has also been discussed (Dumas et al. 2014). Many studies on occupational respiratory hazards related to the presence of irritants in different industries’ workplaces (gases, dust, fumes, mists, vapours, smoke, fog and sprays) were reported in the literature. In some cases, substances are generated via industrial processes, for example during the aeration process, drying of the sludge and mechanical filtering processes.
SO2 is another important air pollutant to monitor in workplaces for worker safety (Goudarzi et al. 2016; Yan et al. 2020; Wang et al. 2022). It is produced from the combustion of solid fossil fuels and is considered the most relevant pollutant from materials’ deterioration, especially in the corrosion of metals and stone recession. These corrosive effects are even greater with the presence of an oxidizer such as NO2. SO2 is the most important pollutant from industrial activities such as petroleum refining, non-ferrous metal smelting and burning of coal for energy production. Exposure to SO2 can result in an increased risk of lung cancer (Lee et al. 2002; Hamra et al. 2015) and heart and respiratory diseases (Golbaz and Jonidi Jafari 2011; Shang et al. 2013; Beelen et al. 2014; Dursun et al. 2015).
In recent years, despite a general improvement in air quality in workplaces, a real concern over the preservation of the health of workers exposed to atmospheric pollution remains (Charlier et al. 2021; Motta et al. 2021; Motta et al. 2022a; Montano et al. 2022; Nascimento et al. 2020; Zhang et al. 2021). Even though several studies regarding the beneficial effects of thermal waters for users are recognized in the literature (Giampaoli et al. 2013; Carbajo and Maraver 2017; Costantino et al. 2020), only one study so far has monitored the air concentration of pollutants such as H2S in thermal spring environments (Fazlzadeh et al. 2018), while no studies have investigated SO2 concentration in the air of this specific workplace. Obviously, the understanding of the origin and the evolution of contaminants is necessary for the decisions that must be taken by industrial companies and international agencies of health (Mohai et al. 2011; Vimercati et al. 2018; Motta et al. 2022b; Pironti et al. 2022).
In this study, we monitored the concentration of specific pollutants (H2S and SO2) at the thermal springs of Contursi Terme (Salerno, Italy) for 4 months to evaluate workers’ exposure to these harmful pollutants and evaluate the need to implement corrective measures to safeguard workers’ health.
Material and method
Materials
All the reagents used for the measurements (Na2CO3, NaHCO3, H2SO4, Na2SO4, H2O2, NaOH, thiosulphate, iodine, certified reference material, silica gel) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Sampling site
Water and air sampling was performed from 21 January to 21 April 2015 in the thermal springs of Contursi Terme (Salerno, Italy). The location of this village in the Region Campania of Italy is shown in Fig. 1.
With a temperature at the source of 47.6 ± 0.5 °C, the thermal spring water at Contursi Terme can be classified as hyperthermal (Štambuk-Giljanović 2008; Quattrini et al. 2016). Monthly water samples were taken from the pool of the thermal springs during the sampling period. Physicochemical characteristics of the thermal water were monitored by measuring the concentration of cations (Li+, Na+, K+, Ca2+ and Mg2+), anions (Cl−, SO42− and HCO3−), silica and sulphide; conductivity; fixed residue at 180 °C; hardness; temperature; and pH at the source and pool. The temperature, conductivity and pH of water samples were determined using a multiparameter probe from Hanna Instruments (HI98194).
Passive and active air monitoring
Air monitoring was conducted using both active and passive samplers. Passive samplers employed were RING® radial diffusive devices (purchased from Aquaria Srl, Milan, Italy) (Cucciniello et al. 2012, 2015; Proto et al. 2014; Motta et al. 2018). Bar and restaurant rooms were considered indoor environments while the external pools as outdoor environments. H2S and SO2 were measured according to the methodologies of the National Institute for Occupational Safety and Health (NIOSH), the US federal agency for research and prevention of work-related injury and illness (Methodology 6013 for Hydrogen Sulphide and 6004 for Sulphur Dioxide) (NIOSH 1994a, b).
Active sampling was carried out near the thermal spring source (1 m away from the thermal source) with a sorbent tube and an AP Buck VSS 1 pump (Aquaria srl, Italy) using an airflow rate of 200 mL/min (Cucciniello et al. 2017). Active monitoring was done two times per month for exposure times of 20–30 min. Passive sampling was performed in the bar and restaurant room (indoors), and external pool (outdoors). For each passive sampler, the start and the end of sampling period coincide with the days in which active sampling was performed, so the exposure time is in the range 7–14 days.
A detailed description of all the collected samples, including sampling date, exposure time, weather conditions and number of samples, is reported in Table S1. For each sample type (SO2 active sampling, H2S active sampling, SO2 passive sampling indoor, SO2 passive sampling outdoor, H2S passive sampling indoor and H2S passive sampling outdoor), samples are enumerated in chronological order from January to April.
The substrate for the H2S monitoring in the air was based on zinc acetate–impregnated silica in glass tubes. After collection, H2S is oxidated to sulphate with an alkaline solution of hydrogen peroxide according to previous work (Motta et al. 2014). Triethanolamine was used as the substrate for SO2, and then water extraction was performed and SO2 was recovered as sulphate.
Chromatographic analyses
Target chemicals (SO2 and H2S) were quantified as sulphate using ion-exchange chromatography on a Thermo Scientific Dionex™ Aquion™ ion chromatograph equipped with a conductivity system detector (Ricciardi et al. 2022). A Dionex IonPac AS23 carbonate eluent anion-exchange column was used for anions (Cl− and SO42−), while a Dionex IonPac CS12A (sulphuric acid as eluent) for cations (Li+, Na+, K+, Ca2+ and Mg2+). Ionic concentrations (expressed as mg/L) were obtained using calibration curves prepared employing standard ion solutions. The precision, expressed as one standard deviation, was 1% for all the ions considered.
Sulphidimetric grade determination
Evaluation of total and dissolved sulphide in water was done according to national protocol APAT IRSA-CNR (APAT IRSA-CNR 2003):
where a is the volume (mL) of the iodine solution used in the titration, b is the volume (mL) of the thiosulphate solution used in the titration, NI is the normality of the iodine solution, NT is the normality of the thiosulphate solution, V is the volume (mL) of the sample taken, and 16 is the equivalent weight of sulphide.
For the determination of the dissolved sulphide content, a preliminary separation of the suspended sulphides by sedimentation was carried out, making them flocculate by the addition of a solution of aluminium chloride and sodium hydroxide.
Statistical analysis
Statistical analyses, including one-way ANOVA (analysis of variance), were performed using the R Studio software (version 4.1.1). In particular, we evaluated the statistical differences between the indoor and outdoor concentrations of the considered pollutants obtained by passive sampling and the statistical differences between the concentrations recorded in different sampling periods. The null hypotheses for the ANOVA were that there are no differences between indoor and outdoor concentrations detected for the same pollutant during the same sampling period and there are no differences between concentrations recorded in different sampling periods. Hence, the independent variables were the “type of environment” (indoor and outdoor) and the “sampling period” (January–February and March–April), whereas the dependent variable was the air concentration of the considered pollutants. The significance level was set at α = 0.05.
Results
The results of the physicochemical analysis of water are summarized in Table 1. The bicarbonate sulphurous mineral thermal water presented concentrations of HCO3− equal to 1800 ± 40 mg/L, SO42− of 270 ± 10 mg/L and a total sulphide content of 28 ± 2 mg/L (dissolved sulphide was 16 ± 1 mg/L).
The atmospheric pollutants (SO2 and H2S) were measured at different times and locations in the thermal spring sites. First, active samples were collected to measure the concentration of pollutants in a short time interval (20–30 min) and in a specific place. Air concentrations of SO2 and H2S obtained by active sampling were in the range 2.0–5.2 mg/m3 and 2.2–20.2 mg/m3, respectively (Table 2). These values are snapshots of the pollutant’s concentrations near its emission source.
To evaluate the mean concentration of a pollutant to which workers are exposed daily and temporal variations, passive sampling was performed both indoors (bar and restaurant room) and outdoors (external pool). The use of passive samplers allows to obtain threshold limit value-time-weighted averages (TLV-TWA) of the concentrations of pollutants in a wider time lapse than active sampler (168–336 h vs 20–30 min) and to acquire average concentrations over time. In fact, contrary to active sampling, the values resulting from passive sampling are not susceptible to punctual emissions and momentary variations of pollutant concentration.
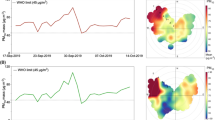
The temporal variation in concentrations measured by passive sampling during the monitoring campaigns is shown in Fig. 2 for SO2 and in Fig. 3 for H2S for indoor (yellow) and outdoor (green) environments. SO2 and H2S concentrations detected by passive sampling are generally lower than those obtained by active sampling and varied from 0.11 to 0.91 mg/m3 and from 0.11 to 1.90 mg/m3, respectively.
The ANOVA showed there are no significant differences (p-value > 0.05) between the indoor and outdoor concentration values for SO2, whereas there are considerable differences (p-value < 0.05) between the indoor and outdoor concentrations for H2S. Moreover, there are significant differences between the concentrations detected during the first half of the sampling period—January to February—and those recorded in the second half—March to April—for SO2 (p-value < 0.05), but not for H2S (p-value > 0.05).
Discussion
Because the effects of pollutant exposure on human health can become visible only after several years, when the action measures are necessary but also difficult to take, a prevention strategy is crucial to protect human health in workplaces, where people spend most of their daily time. The importance of this study is linked to the determination at this thermal site of the presence and levels of certain types of pollutants that, even at low concentrations, can have lasting harmful effects on human health over time.
In this study, we looked at two pollutants that are most representative of thermal water composition, H2S and SO2. To obtain a full overview of the concentration of pollutants at this thermal site, both active and passive air sampling were performed. For both pollutants, the concentrations detected by active sampling (Table 2) were higher than those recorded by passive sampling (Table S2 and Figs. 2 and 3). This is in line with the principle that active sampling gives a point measure of concentration and not a time average. Moreover, active sampling was carried out near the thermal spring source (at the entrances of the hot water into the pool), whereas passive samplers were placed in the most crowded places with customers and workers, near the pool and the workstations to better evaluate the concentration at which customers/patients and/or workers are exposed.
During the monitoring campaigns, we noted a decreasing trend in the concentration of SO2 (Fig. 2) from January to February (means of 0.65 ± 0.04 mg/m3 for outdoor and 0.7 ± 0.2 mg/m3 for indoor) to March–April (means of 0.2 ± 0.1 mg/m3 for outdoor and 0.3 ± 0.1 mg/m3 for indoor), without significant differences between the indoor and outdoor environments (p-value > 0.05). The presence of this pollutant is mainly related to anthropogenic emissions from industries and household heating, and not to the thermal spring itself.
The air concentration values for SO2 obtained by passive sampling were always lower than the exposure limits in workplaces, expressed as both TLV-TWA (threshold limit value-time-weighted average), that is 5.2 mg/m3, and TLV-STEL (threshold limit value-short-term exposure limit), that is 13.3 mg/m3. Conversely, active sampling showed air concentrations closer to the TLV-TWA limit. In literature, studies involving exercising asthmatics indicate that a proportion of the population experience changes in pulmonary function and respiratory symptoms after periods of exposure to SO2 as short as 10 min. For instance, in Canada an increase of 11% in hospitalizations due to respiratory diseases from 1995 to 2000 in children from 0 to 14 years was reported after exposure to 10 μg/m3 of sulphur dioxide (Li et al. 2019). Based on this evidence, the World Health Organization (WHO) air quality guidelines revised the SO2 guideline, adopting a prudent precautionary limit of 20 µg/m3 for 24-h periods and a value of 500 µg/m3 for 10-min averages (World Health Organization 2006). Moreover, industrial activities, e.g. oil and gas extraction, contribute to the increase of air concentration of H2S and SO2 in rural areas (Burstyn et al. 2007). In Italy, regulatory limits are set to 350 µg/m3 as the hourly average (not to be exceeded more than 24 times per calendar year) and 125 µg/m3 for daily averages (not to be exceeded more than 3 times per calendar year) (D.Lgs 155/2010 2010). All the recorded concentrations of SO2 in this study exceeded these national limits.
In these thermal-mineral springs, various effects are present at the same time: water, high humidity and temperature. These conditions promoted SO2 solubility in water to give an acidic solution, capable of reacting with other chemical compounds present in that environment. These representative pollutants are strongly linked to an increase in temperature and visitors in correspondence to spring. Moreover, the indoor environments (reception, bar, restaurant room etc.) were characterized by the presence in the air of acidic pollutants such as SO2 and H2S that cause corrosion of metals present in electronic devices, resulting in faster degradation of the indoor environments. Both SO2 and H2S are strongly corrosive agents, so their presence in indoor air must be monitored to prevent the degradation of metal-containing devices (Cox and Lyon 1994; Kobus 2000; Wen et al. 2018).
On the other hand, in the case of H2S (Fig. 3), no significant variation of concentrations was noticed between the sampling period of January–February and that of March–April (p-value > 0.05), while a significant difference between indoor (overall mean of 0.23 ± 0.05 mg/m3) and outdoor (overall mean of 1.0 ± 0.5 mg/m3) concentrations was observed (p-value < 0.05). This is representative of the fact that the presence of H2S is specific to the thermal springs.
European and national legislation do not define limit values or air quality target values for H2S. In the absence of specific regulatory references, it is standard practice to refer to the WHO guideline values. The atmospheric concentration limit values are 7 ppm (9.76 mg/m3) for a 30-min average to olfactory pollution and 150 ppm (209 mg/m3) for a daily average to prevent eye irritation (World Health Organization 2003). Furthermore, in Europe the Scientific Committee on Occupational Exposure Limits (SCOEL) recommended a TLV-TWA of 5 ppm (7 mg/m3) and a TLV-STEL of 10 ppm (14 mg/m3) (SCOEL/SUM/124 2007 2007; Elwood 2021). Air concentrations of H2S from 0.2 to 29.4 ppm (0.3–41 mg/m3), noticeably higher than these TWA and STEL, were detected in thermal springs located in Ardabil Province, in a structure with several indoor pools (Fazlzadeh et al. 2018). The thermal site of Contursi Terme is characterized by outdoor pools only, so lower concentrations of H2S in the air are expected. In fact, passive sampling allowed us to detect concentrations ranging from 0.11 to 1.90 mg/m3 (Fig. 3) and these values present no risks for human health. These results can be explained by the fact that the air concentration of H2S is strongly related to the thermal spring emissions, while that of SO2 is probably derived also by other emission pathways.
However, active sampling performed near the spring source revealed that concentrations were, in some cases, higher than the limits in the working environment for both H2S and SO2.
This study has some limitations. First, the investigation is limited to monitoring a single site that has specific microclimate conditions and structural elements that are different from those of other thermal natural springs. Second, this study ruled out some factors that can affect local employee health during work hours in a thermal spring such as dietary habits, job description, lifestyle and smoking. Furthermore, numerous workers’ psychological, physical and health conditions were not included in the data collection. However, in this study, we focused on assessing the air quality, in terms of H2S and SO2 concentrations, to which workers at this spa complex are daily exposed. We believe that this approach is the first stage in safeguarding the health of the workers and to which should be given more consideration. To our knowledge, only one other example of H2S concentration monitoring in a spa area can be found in the literature. Undoubtedly, the above limitations must be taken into account in a quantitative assessment of the health effects on workers as a result of exposure to these pollutants.
Conclusion
The chemical identification of pollutants in a particular environment, such as a thermal spring, and control of their concentration are essential to suggest new and improved procedures of safety and guidelines for professional activities. Although the water of thermal structures provides beneficial effects on human health, air monitoring, performed near the spring source, showed that concentrations of sulphurous compounds (both H2S and SO2) are in some cases higher than the limits in the working environment. Therefore, further investigations and regulations are needed to estimate the occupational risk and ensure workers’ safety in these particular working places.
Data availability
Not applicable.
References
APAT, IRSA-CNR (2003) Metodi analitici per le acque. In: Manuali e Linee guida n. 29/2003, 3, pp 1153
Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR (2006) Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res 4:9–14. https://doi.org/10.1158/1541-7786.MCR-05-0126
Beelen R, Stafoggia M, Raaschou-Nielsen O et al (2014) Long-term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology 25:368–378
Burstyn I, Senthilselvan A, Kim H-M et al (2007) Industrial sources influence air concentrations of hydrogen sulfide and sulfur dioxide in rural areas of Western Canada. J Air Waste Manag Assoc 57:1241–1250. https://doi.org/10.3155/1047-3289.57.10.1241
Carbajo JM, Maraver F (2017) Sulphurous mineral waters: new applications for health. Evid-Based Complement Altern Med 2017:e8034084. https://doi.org/10.1155/2017/8034084
Charlier B, Coglianese A, De Rosa F et al (2021) Chemical risk in hospital settings: overview on monitoring strategies and international regulatory aspects. J Public Health Res 10:1993. https://doi.org/10.4081/jphr.2021.1993
Costantino M, Izzo V, Conti V et al (2020) Sulphate mineral waters: a medical resource in several disorders. J Tradit Complement Med 10:320–326. https://doi.org/10.1016/j.jtcme.2019.04.004
Cox A, Lyon SB (1994) An electrochemical study of the atmospheric corrosion of mild steel—III. The effect of sulphur dioxide. Corros Sci 36:1193–1199. https://doi.org/10.1016/0010-938X(94)90143-0
Croutier AL (1992) Taking the waters: spirit, art, sensuality. Abbeville Press, New York
Cucciniello R, Proto A, Alfano D, Motta O (2012) Synthesis, characterization and field evaluation of a new calcium-based CO2 absorbent for radial diffusive sampler. Atmos Environ 60:82–87. https://doi.org/10.1016/j.atmosenv.2012.06.023
Cucciniello R, Proto A, La Femina R et al (2017) A new sorbent tube for atmospheric NOx determination by active sampling. Talanta 164:403–406. https://doi.org/10.1016/j.talanta.2016.12.006
Cucciniello R, Proto A, Rossi F et al (2015) An improved method for BTEX extraction from charcoal. Anal Methods 7:4811–4815. https://doi.org/10.1039/C5AY00828J
D.Lgs 155/2010 (2010) Attuazione della direttiva 2008/50/CE relativa alla qualità dell’aria ambiente e per un’aria più pulita in Europa
Dumas O, Laurent E, Bousquet J et al (2014) Occupational irritants and asthma: an Estonian cross-sectional study of 34,000 adults. Eur Respir J 44:647–656. https://doi.org/10.1183/09031936.00172213
Dursun S, Kunt F, Taylan O (2015) Modelling sulphur dioxide levels of Konya City using artificial intelligent related to ozone, nitrogen dioxide and meteorological factors. Int J Environ Sci Technol 12:3915–3928. https://doi.org/10.1007/s13762-015-0821-2
Elwood M (2021) The scientific basis for occupational exposure limits for hydrogen sulphide—a critical commentary. Int J Environ Res Public Health 18:2866. https://doi.org/10.3390/ijerph18062866
EPA (1986) Health assessment document for hydrogen sulfide. US Environmental Protection Agency, Washington, DC
Fazlzadeh M, Rostami R, Norouzian Baghani A et al (2018) Hydrogen sulfide concentrations in indoor air of thermal springs. Hum Ecol Risk Assess Int J 24:1441–1452. https://doi.org/10.1080/10807039.2017.1413537
Frosch WA (2007) “Taking the waters”—springs, wells, and spas. FASEB J 21:1948–1950. https://doi.org/10.1096/fj.07-0702ufm
Frost GJ (2004) The spa as a model of an optimal healing environment. J Altern Complement Med 10:S-85. https://doi.org/10.1089/acm.2004.10.S-85
Ghantous Y, Yaffi V, Abu-Elnaaj I (2015) Oral cavity cancer: epidemiology and early diagnosis. Refuat Hapeh Vehashinayim (1993) 32(55–63):71
Giampaoli S, Valeriani F, Gianfranceschi G et al (2013) Hydrogen sulfide in thermal spring waters and its action on bacteria of human origin. Microchem J 108:210–214. https://doi.org/10.1016/j.microc.2012.10.022
Golbaz S, Jonidi Jafari A (2011) A comparative study of health quality of air in Tehran and Isfahan; 2008–2009. Razi J Med Sci 18:28–46
Goudarzi G, Geravandi S, Idani E et al (2016) An evaluation of hospital admission respiratory disease attributed to sulfur dioxide ambient concentration in Ahvaz from 2011 through 2013. Environ Sci Pollut Res 23:22001–22007. https://doi.org/10.1007/s11356-016-7447-x
Hamra GB, Laden F, Cohen AJ et al (2015) Lung cancer and exposure to nitrogen dioxide and traffic: a systematic review and meta-analysis. Environ Health Perspect 123:1107–1112. https://doi.org/10.1289/ehp.1408882
IARC (1992) Occupational exposures to mists and vapours from strong inorganic acids; and other industrial chemicals. In: IARC monographs on the evaluation of carcinogenic risks to humans, N° 54. International Agency for Research on Cancer, Lyon, FR
Jackson R (1990) Waters and spas in the classical world. Med Hist 34:1–13. https://doi.org/10.1017/S0025727300070952
Kobus J (2000) Long-term atmospheric corrosion monitoring. Mater Corros 51:104–108. https://doi.org/10.1002/(SICI)1521-4176(200002)51:2%3c104::AID-MACO104%3e3.0.CO;2-V
Lee WJ, Teschke K, Kauppinen T et al (2002) Mortality from lung cancer in workers exposed to sulfur dioxide in the pulp and paper industry. Environ Health Perspect 110:991–995. https://doi.org/10.1289/ehp.02110991
Legator MS, Singleton C (1997) Panel on hydrogen sulfide. American PublicHealth Association’s annual meeting, Indianapolis
Li R, Fu H, Cui L et al (2019) The spatiotemporal variation and key factors of SO2 in 336 cities across China. J Clean Prod 210:602–611. https://doi.org/10.1016/j.jclepro.2018.11.062
Lim E, Mbowe O, Lee ASW, Davis J (2016) Effect of environmental exposure to hydrogen sulfide on central nervous system and respiratory function: a systematic review of human studies. Int J Occup Environ Health 22:80–90. https://doi.org/10.1080/10773525.2016.1145881
Mohai P, Kweon B-S, Lee S, Ard K (2011) Air pollution around schools is linked to poorer student health and academic performance. Health Aff 30:852–862. https://doi.org/10.1377/hlthaff.2011.0077
Montano L, Pironti C, Pinto G, Ricciardi M, Buono A, Brogna C, Venier M, Piscopo M, Amoresano A, Motta O (2022) Polychlorinated biphenyls (PCBs) in the environment: occupational and exposure events, effects on human health and fertility. Toxics 10:365–386. https://doi.org/10.3390/toxics10070365
Motta O, Cucciniello R, La Femina R et al (2018) Development of a new radial passive sampling device for atmospheric NOx determination. Talanta 190:199–203. https://doi.org/10.1016/j.talanta.2018.07.088
Motta O, Cucciniello R, Scicali C, Proto A (2014) A study on the applicability of zinc acetate impregnated silica substrate in the collection of hydrogen sulfide by active sampling. Talanta 128:268–272. https://doi.org/10.1016/j.talanta.2014.04.031
Motta O, Pironti C, Ricciardi M et al (2022a) Leonardo da Vinci’s “Last Supper”: a case study to evaluate the influence of visitors on the museum preservation systems. Environ Sci Pollut Res 29:29391–29398. https://doi.org/10.1007/s11356-021-13741-9
Motta O, Charlier B, De Caro F et al (2021) Environmental and biological monitoring of formaldehyde inside a hospital setting: a combined approach to manage chemical risk in workplaces. J Public Health Res 10:2012. https://doi.org/10.4081/jphr.2021.2012
Motta O, Pironti C, Venier M, Proto A (2022b) An innovative filtering system for the handling of asbestos-based products: improvement of safety and quality of work in analysis laboratories. Toxics 10:281. https://doi.org/10.3390/toxics10060281
Nascimento AP, Santos JM, Mill JG et al (2020) Association between the incidence of acute respiratory diseases in children and ambient concentrations of SO2, PM10 and chemical elements in fine particles. Environ Res 188:109619. https://doi.org/10.1016/j.envres.2020.109619
NIOSH (1994a) “Method 6013: hydrogen sulfide”, NIOSH manual of analytical methods, Fourth Edition. DHHS (NIOSH) Publication, Washington, DC
NIOSH (1994b) “Method 6004: sulfur dioxide”, NIOSH manual of analytical methods, Fourth Edition. DHHS (NIOSH) Publication, Washington, DC
NTP (2016) 14th report on carcinogens. In: National Toxicology Program (NTP). Research Triangle Park, Department of Health and Human Services, Public Health Service
Nuvolone D, Petri D, Pepe P, Voller F (2019) Health effects associated with chronic exposure to low-level hydrogen sulfide from geothermoelectric power plants. A residential cohort study in the geothermal area of Mt. Amiata in Tuscany. Sci Total Environ 659:973–982. https://doi.org/10.1016/j.scitotenv.2018.12.363
Pironti C, Ricciardi M, Motta O et al (2021a) Microplastics in the environment: intake through the food web, human exposure and toxicological effects. Toxics 9:224. https://doi.org/10.3390/toxics9090224
Pironti C, Ricciardi M, Proto A et al (2021b) Endocrine-disrupting compounds: an overview on their occurrence in the aquatic environment and human exposure. Water 13:1347. https://doi.org/10.3390/w13101347
Pironti C, Ricciardi M, Proto A et al (2022) New analytical approach to monitoring air quality in historical monuments through the isotopic ratio of CO2. Environ Sci Pollut Res 29:29385–29390. https://doi.org/10.1007/s11356-020-12215-8
Proto A, Cucciniello R, Rossi F, Motta O (2014) Stable carbon isotope ratio in atmospheric CO2 collected by new diffusive devices. Environ Sci Pollut Res 21:3182–3186. https://doi.org/10.1007/s11356-013-2369-3
Quattrini S, Pampaloni B, Brandi ML (2016) Natural mineral waters: chemical characteristics and health effects. Clin Cases Miner Bone Metab 13:173–180. https://doi.org/10.11138/ccmbm/2016.13.3.173
Rafieepour A, Gholamzadeh N, Haj Ghasemkhan A (2013) The effect of the use of NP305 masks in improving respiratory symptoms in workers exposed to sulfuric acid mists in plating and pickling units. Electron Physician 616–622. https://doi.org/10.14661/2013.616-622
Ricciardi M, Pironti C, Motta O et al (2021) Microplastics in the aquatic environment: occurrence, persistence, analysis, and human exposure. Water 13:973. https://doi.org/10.3390/w13070973
Ricciardi M, Pironti C, Motta O et al (2022) Investigations on historical monuments’ deterioration through chemical and isotopic analyses: an Italian case study. Environ Sci Pollut Res 29:29409–29418. https://doi.org/10.1007/s11356-021-15103-x
Routh HB, Bhowmik KR, Parish LC, Witkowski JA (1996) Balneology, mineral water, and spas in historical perspective. Clin Dermatol 14:551–554. https://doi.org/10.1016/S0738-081X(96)00083-1
SCOEL (2007) Recommendation from the Scientific Committee on Occupational Exposure Limits for Hydrogen Sulphide. Scientific Committee on Occupational Exposure Limits (SCOEL). SCOEL/SUM/124 2007. European Commision
Shang Y, Sun Z, Cao J et al (2013) Systematic review of Chinese studies of short-term exposure to air pollution and daily mortality. Environ Int 54:100–111. https://doi.org/10.1016/j.envint.2013.01.010
Štambuk-Giljanović N (2008) Characteristics and origin of the hydrogen sulphide spring water from the Split spa (Southern Croatia). Environ Monit Assess 140:109–117. https://doi.org/10.1007/s10661-007-9852-6
Stanhope J, Weinstein P, Cook A (2017) Do natural spring waters in Australia and New Zealand affect health? A systematic review. J Water Health 16:1–13. https://doi.org/10.2166/wh.2017.209
Torres-Ceron DA, Acosta-Medina CD, Restrepo-Parra E (2019) Geothermal and mineralogic analysis of hot springs in the Puracé-La Mina Sector in Cauca. Colombia Geofluids 2019:e3191454. https://doi.org/10.1155/2019/3191454
Valeriani F, Margarucci LM, Romano Spica V (2018) Recreational use of spa thermal waters: criticisms and perspectives for innovative treatments. Int J Environ Res Public Health 15:2675. https://doi.org/10.3390/ijerph15122675
Vianna MIP, Santana VS, McKelvey W (2005) Periodontal health and oral mucosal lesions as related to occupational exposure to acid mists. Commun Dent Oral Epidemiol 33:341–348. https://doi.org/10.1111/j.1600-0528.2005.00226.x
Vimercati L, Cavone D, Maria LD et al (2018) 1579 Environmental asbestos exposure in southern Italy: mesothelioma cases due to the same pollution source. Occup Environ Med 75:A142–A143. https://doi.org/10.1136/oemed-2018-ICOHabstracts.404
Wang X-Q, Zhao J-W, Zhang K-D et al (2022) Short-term effect of sulfur dioxide (SO2) change on the risk of tuberculosis outpatient visits in 16 cities of Anhui Province, China: the first multi-city study to explore differences in occupational patients. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-19438-x
Wen X, Bai P, Luo B et al (2018) Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment. Corros Sci 139:124–140. https://doi.org/10.1016/j.corsci.2018.05.002
World Health Organization (2006). Occupational and Environmental Health Team (2006) WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005: summary of risk assessment. World Health Organization
World Health Organization (2003). Satety International Programme on Chemical CHSJ (2003) Hydrogen sulfide: human health aspects. World Health Organization
Yan M, Li C, Zhang L et al (2020) Association between long-term exposure to sulfur dioxide pollution and hypertension incidence in northern China: a 12-year cohort study. Environ Sci Pollut Res 27:21826–21835. https://doi.org/10.1007/s11356-020-08572-z
Zhang X, Wang Z, Cheng M et al (2021) Long-term ambient SO2 concentration and its exposure risk across China inferred from OMI observations from 2005 to 2018. Atmos Res 247:105150. https://doi.org/10.1016/j.atmosres.2020.105150
Funding
Open access funding provided by Università degli Studi di Salerno within the CRUI-CARE Agreement. This work was financially supported by Fondi di Ateneo per la Ricerca di Base (FARB 2019), University of Salerno.
Author information
Authors and Affiliations
Contributions
Conceptualization: OM, AP. Data curation: CP, AF, MR. Formal analysis: CP, RC, AF, MR. Funding acquisition: OM, AP. Investigation: CP, MR, RC, AF. Methodology: AF, RC, CP, MR. Project administration and resource: OM, AP. Software: CP, MR, AF. Supervision and validation: RC, OM, AP, MV. Visualization: CP, MR, RC, AF, OM, AP, MV. Writing—original draft: CP, MR. Writing—review and editing: OM, AP, MV.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pironti, C., Ricciardi, M., Motta, O. et al. Sulphurous air pollutants and exposure events of workers in thermal-mineral springs: a case study of Contursi Terme (Salerno, Italy). Environ Sci Pollut Res 30, 3112–3120 (2023). https://doi.org/10.1007/s11356-022-22432-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22432-y