Abstract
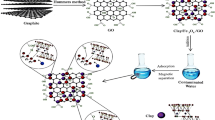
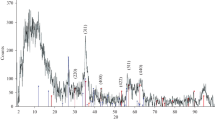
The muscovite mica clay-graphene oxide-maghemite-magnetite (γ-Fe2O3-Fe3O4) composite was first used for the adsorption of caesium(I) and cobalt(II). The presence of clay minerals, graphene oxide, maghemite, and magnetite was detected in the prepared composite by XRD, WD-XRF, Mössbauer spectroscopy, and ATR-FTIR. The SEM and TEM results show that the composite has a layered structure with irregularly shaped pores on the surface. It was found that the adsorption of ions depends on the initial concentration, pH (except for caesium), mass of adsorbent, temperature, and contact time. The maximum adsorption capacity for Cs(I) and Co(II) was 2286 mg/g and 652 mg/g, respectively, and was obtained at concentrations (Cs(I) = 12,630 mg/L; Co(II) = 3200 mg/L), adsorbent mass of 0.01 g, pH (Cs(I) = 7; Co(II) = 5), temperature of 20 ± 1 °C, and contact time of 24 h. The high adsorption capacity of the composite could be due to a diversity of functional groups, a large number of active sites or the multilayer adsorption of caesium and cobalt ions on the surface of the composite. The Freundlich, Langmuir isotherms, and the pseudo-second-order kinetic model better describe the adsorption of these ions on the composite. The adsorption was non-spontaneous endothermic for Cs(I) and spontaneous endothermic for Co(II). The proposed mechanism of adsorption of Cs and Co ions on the composite is complex and involves electrostatic interactions and ion exchange. The ANFIS model proved to be quite effective in predicting the adsorption of Cs(I) and Co(II), as shown by the obtained values of R2, MSE, SSE, and ARE.
Graphical abstract











Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abdel Maksoud MIA, Sami NM, Hassan HS et al (2022) Novel adsorbent based on carbon-modified zirconia/spinel ferrite nanostructures: evaluation for the removal of cobalt and europium radionuclides from aqueous solutions. J Colloid Interface Sci 607:111–124. https://doi.org/10.1016/J.JCIS.2021.08.166
Achour S, Amokrane S, Chegrouche S, et al (2021) Adsorption mechanism study of radionuclide 60Co by purified and α-Fe2O3-supported bentonite from radioactive solution. Arab J Sci Eng 1–17. https://doi.org/10.1007/S13369-021-05570-2
Adibmehr Z (2020) Faghihian H (2020) Preparation of highly selective magnetic cobalt ion-imprinted polymer based on functionalized SBA-15 for removal Co2+ from aqueous solutions. J Environ Heal Sci Eng 172(17):1213–1225. https://doi.org/10.1007/S40201-019-00439-X
Al-Musawi TJ, Mengelizadeh N, Al Rawi O (2021) Balarak D (2021) Capacity and modeling of acid blue 113 dye adsorption onto chitosan magnetized by Fe2O3 nanoparticles. J Polym Environ 301(30):344–359. https://doi.org/10.1007/S10924-021-02200-8
Ain QU, Zhang H, Yaseen M et al (2020) Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb(II), Cd(II), and crystal violet from aqueous solution. J Clean Prod 247:119088. https://doi.org/10.1016/J.JCLEPRO.2019.119088
Awang NA, Salleh WNW, Alisah MFM et al (2019) Adsorption of cesium from aqueous solution using chitosan beads. J Teknol 81:135–140. https://doi.org/10.11113/JT.V81.11514
Belachew N, Bekele G (2019) Synergy of magnetite intercalated bentonite for enhanced adsorption of congo red dye. SILICON 12(3):603–612. https://doi.org/10.1007/S12633-019-00152-2
Belcaid A, Beakou BH, El Hassani K et al (2021) Efficient removal of Cr(VI) and Co(II) from aqueous solution by activated carbon from Manihot esculenta Crantz agricultural bio-waste. Water Sci Technol 83:556–566. https://doi.org/10.2166/WST.2020.585
Brix K, Hein C, Haben A, Kautenburger R (2019) Adsorption of caesium on raw Ca-bentonite in high saline solutions: influence of concentration, mineral composition, other radionuclides and modelling. Appl Clay Sci 182:105275. https://doi.org/10.1016/J.CLAY.2019.105275
Ca DV, Cox JA (2004) Solid phase extraction of cesium from aqueous solution using sol-gel encapsulated cobalt hexacyanoferrate. Microchim Acta 147:31–37. https://doi.org/10.1007/S00604-004-0224-4
Chen S, Brown L, Levendorf M et al (2011) Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 5:1321–1327. https://doi.org/10.1021/NN103028D
Compeán-Jasso ME, Ruiz F, Martínez JR, Herrera-Gómez A (2008) Magnetic properties of magnetite nanoparticles synthesized by forced hydrolysis. Mater Lett 62:4248–4250. https://doi.org/10.1016/J.MATLET.2008.06.053
Damayanti NP (2010) Preparation of Superhydrophobic PET fabric from Al2O3-SiO2 hybrid: geometrical approach to create high contact angle surface from low contact angle materials. J Sol-Gel Sci Technol 56:47–52. https://doi.org/10.1007/s10971-010-2271-0
Dolatabadi M, Mehrabpour M, Esfandyari M et al (2018) Modeling of simultaneous adsorption of dye and metal ion by sawdust from aqueous solution using of ANN and ANFIS. Chemom Intell Lab Syst 181:72–78. https://doi.org/10.1016/J.CHEMOLAB.2018.07.012
Efimova NV, Krasnopyorova AP, Yuhno GD et al (2021) Uptake of radionuclides 60Co, 137Cs, and 90Sr with α-Fe2O3 and Fe3O4 particles from aqueous environment. Mater 14:2899 14-2899. https://doi.org/10.3390/MA14112899
Egbosiuba TC, Abdulkareem AS, Kovo AS et al (2020) Ultrasonic enhanced adsorption of methylene blue onto the optimized surface area of activated carbon: adsorption isotherm, kinetics and thermodynamics. Chem Eng Res Des 153:315–336. https://doi.org/10.1016/J.CHERD.2019.10.016
El-Sayed AA, Hamed MM, Hmmad HA, El-Reefy S (2007) Collection/concentration of trace uranium for spectrophotometric detection using activated carbon and first-derivative spectrophotometry. Radiochim Acta 95:43–48. https://doi.org/10.1524/ract.2007.95.1.43
El-Din AMS, Monir T (2019) Sayed MA (2019) Nano-sized Prussian blue immobilized costless agro-industrial waste for the removal of cesium-137 ions. Environ Sci Pollut Res 2625(26):25550–25563. https://doi.org/10.1007/S11356-019-05851-2
Esmaeili H, Tamjidi S (2020) Ultrasonic-assisted synthesis of natural clay/Fe3O4/graphene oxide for enhance removal of Cr(VI) from aqueous media. Environ Sci Pollut Res 27:31652–31664. https://doi.org/10.1007/S11356-020-09448-Y
Fan QH, Tanaka M, Tanaka K et al (2014) An EXAFS study on the effects of natural organic matter and the expandability of clay minerals on cesium adsorption and mobility. Geochim Cosmochim Acta 135:49–65. https://doi.org/10.1016/J.GCA.2014.02.049
Foroutan R, Peighambardoust SJ, Mohammadi R et al (2020) Influence of chitosan and magnetic iron nanoparticles on chromium adsorption behavior of natural clay: adaptive neuro-fuzzy inference modeling. Int J Biol Macromol 151:355–365. https://doi.org/10.1016/J.IJBIOMAC.2020.02.202
Fuller AJ, Shaw S, Peacock CL et al (2014) Ionic strength and pH dependent multi-site sorption of Cs onto a micaceous aquifer sediment. Appl Geochemistry 40:32–42. https://doi.org/10.1016/j.apgeochem.2013.10.017
Garty G, Xu Y, Johnson GW et al (2020) (2020) VADER: a variable dose-rate external 137Cs irradiator for internal emitter and low dose rate studies. Sci Reports 101(10):1–12. https://doi.org/10.1038/s41598-020-76941-2
Haddad D, Mellah A, Nibou D, Khemaissia S (2018) Promising enhancement in the removal of uranium ions by surface-modified activated carbons: kinetic and equilibrium studies. J Environ Eng 144:04018027. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001349
He M, Zhu Y, Yang Y et al (2011) Adsorption of cobalt(II) ions from aqueous solutions by palygorskite. Appl Clay Sci 54:292–296. https://doi.org/10.1016/j.clay.2011.09.013
Hofmann S, Voïtchovsky K, Spijker P et al (2016) (2016) Visualising the molecular alteration of the calcite (104) – water interface by sodium nitrate. Sci Reports 61(6):1–11. https://doi.org/10.1038/srep21576
Hoor YQ, Au PI, Mubarak NM et al (2020) Surface force arising from adsorbed graphene oxide in kaolinite suspensions. Colloids Surfaces A Physicochem Eng Asp 592:124592. https://doi.org/10.1016/J.COLSURFA.2020.124592
Hu B, Ai Y, Jin J et al (2020) Efficient elimination of organic and inorganic pollutants by biochar and biochar-based materials. Biochar 2:47–64. https://doi.org/10.1007/S42773-020-00044-4
Huo J, Yu G, Wang J (2021) Selective adsorption of cesium(I) from water by Prussian blue analogues anchored on 3D reduced graphene oxide aerogel. Sci Total Environ 761:143286. https://doi.org/10.1016/J.SCITOTENV.2020.143286
IAEA (International Atomic Energy Agency) (2006) Environmental consequences of the chernobyl accident and their remediation: twenty years of experience. Report of the Chernobyl Forum Expert Group ‘Environment’. International Atomic Energy Agency Vienna, Austria. https://www.iaea.org/publications/7382/environmental-consequences-of-the-chernobyl-accident-and-their-remediation-twenty-years-of-experience Accessed 30 October 2021
Ibrahim AB, Abass MR, EL-Masry EH, Abou-Mesalam MM (2021) Gamma radiation-induced polymerization of polyacrylic acid-dolomite composite and applications for removal of cesium, cobalt, and zirconium from aqueous solutions. Appl Radiat Isot 178:109956. https://doi.org/10.1016/J.APRADISO.2021.109956
Inyinbor AA, Adekola FA, Olatunji GA (2016) Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour Ind 15:14–27. https://doi.org/10.1016/J.WRI.2016.06.001
Jalali-Rad R, Ghafourian H, Asef Y et al (2004) Biosorption of cesium by native and chemically modified biomass of marine algae: introduce the new biosorbents for biotechnology applications. J Hazard Mater 116:125–134. https://doi.org/10.1016/J.JHAZMAT.2004.08.022
Jang J, Lee DS (2018) Magnetite nanoparticles supported on organically modified montmorillonite for adsorptive removal of iodide from aqueous solution: optimization using response surface methodology. Sci Total Environ 615:549–557. https://doi.org/10.1016/J.SCITOTENV.2017.09.324
Jolivet JP, Chanéac C, Tronc E (2004) Iron oxide chemistry. From molecular clusters to extended solid networks. Chem Commun 4:481–483. https://doi.org/10.1039/B304532N
Kaveeshwar AR, Ponnusamy SK, Revellame ED et al (2018) Pecan shell based activated carbon for removal of iron(II) from fracking wastewater: adsorption kinetics, isotherm and thermodynamic studies. Process Saf Environ Prot 114:107–122. https://doi.org/10.1016/J.PSEP.2017.12.007
Khandaker S, Toyohara Y, Kamida S, Kuba T (2018) Adsorptive removal of cesium from aqueous solution using oxidized bamboo charcoal. Water Resour Ind 19:35–46. https://doi.org/10.1016/J.WRI.2018.01.001
Kryuchkova M, Fakhrullin R (2018) Kaolin alleviates graphene oxide toxicity. Environ Sci Technol Lett 5:295–300. https://doi.org/10.1021/acs.estlett.8b00135
Latrille C, Bildstein O (2022) Cs selectivity and adsorption reversibility on Ca-illite and Ca-vermiculite. Chemosphere 288:132582. https://doi.org/10.1016/J.CHEMOSPHERE.2021.132582
Launer PJ, Arkles B (2013) Infrared analysis of organosilicon compounds: spectra-structure relationships. Silicon Compounds: Silanes and Silicones (eds Arkles B, Larson G):175-178. 10.5281/ZENODO.3696063
Li H, He B, Li P et al (2019) (2019) Adsorption behaviors of Eu(III) on granite: batch, electron probe micro-analysis and modeling studies. Environ Earth Sci 788(78):1–9. https://doi.org/10.1007/S12665-019-8170-Y
Li Y, Sheng G, Sheng J (2014) Magnetite decorated graphene oxide for the highly efficient immobilization of Eu(III) from aqueous solution. J Mol Liq 199:474–480. https://doi.org/10.1016/J.MOLLIQ.2014.08.009
Lingamdinne LP, Choi YL, Kim IS et al (2017) Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides. J Hazard Mater 326:145–156. https://doi.org/10.1016/J.JHAZMAT.2016.12.035
Liu H, Xie S, Liao J et al (2018) Novel graphene oxide/bentonite composite for uranium(VI) adsorption from aqueous solution. J Radioanal Nucl Chem 317:1349–1360. https://doi.org/10.1007/S10967-018-5992-0
Lujanienė G, Beneš P, Štamberg K et al (2010) Effect of natural clay components on sorption of Cs, Pu and Am by the clay. J Radioanal Nucl Chem 286:353–359. https://doi.org/10.1007/s10967-010-0726-y
Macht F, Eusterhues K, Pronk GJ, Totsche KU (2011) Specific surface area of clay minerals: comparison between atomic force microscopy measurements and bulk-gas (N2) and -liquid (EGME) adsorption methods. Appl Clay Sci 53:20–26. https://doi.org/10.1016/J.CLAY.2011.04.006
Makarchuk OV, Dontsova TA, Astrelin IM (2016) Magnetic nanocomposites as efficient sorption materials for removing dyes from aqueous solutions. Nanoscale Res Lett 11:1–7. https://doi.org/10.1186/s11671-016-1364-2
Maneechakr P, Karnjanakom S (2021) Facile utilization of magnetic MnO2@Fe3O4@sulfonated carbon sphere for selective removal of hazardous Pb(II) ion with an excellent capacity: adsorption behavior/isotherm/kinetic/thermodynamic studies. J Environ Chem Eng 9:106191. https://doi.org/10.1016/J.JECE.2021.106191
Marcano DC, Kosynkin DV, Berlin JM et al (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/NN1006368
Masoudi P, Le Coz M, Cazala C, Saito K (2019) Spatial properties of soil analyses and airborne measurements for reconnaissance of soil contamination by 137Cs after Fukushima nuclear accident in 2011. J Environ Radioact 202:74–84. https://doi.org/10.1016/j.jenvrad.2018.11.014
Mejia-Santillan ME, Pariona N, Bravo-C J et al (2018) Physical and arsenic adsorption properties of maghemite and magnetite sub-microparticles. J Magn Magn Mater 451:594–601. https://doi.org/10.1016/J.JMMM.2017.11.111
Mnasri-Ghnimi S, Frini-Srasra N (2019) Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl Clay Sci 179:105151. https://doi.org/10.1016/j.clay.2019.105151
Mookherjee M, Redfern SAT (2002) A high-temperature Fourier transform infrared study of the interlayer and Si–O-stretching region in phengite-2M1. Clay Miner 37:323–336. https://doi.org/10.1180/0009855023720036
Mohseni-Bandpei A, Eslami A, Kazemian H et al (2020) A high density 3-aminopropyltriethoxysilane grafted pumice-derived silica aerogel as an efficient adsorbent for ibuprofen: characterization and optimization of the adsorption data using response surface methodology. Environ Technol Innov 18:100642. https://doi.org/10.1016/J.ETI.2020.100642
Mukai H, Tamura K, Kikuchi R et al (2018) Cesium desorption behavior of weathered biotite in Fukushima considering the actual radioactive contamination level of soils. J Environ Radioact 190–191:81–88. https://doi.org/10.1016/J.JENVRAD.2018.05.006
Narimannejad S, Zhang B (2019) Lye L (2019) Adsorption behavior of cobalt onto saline soil with/without a biosurfactant: kinetic and isotherm studies. Water, Air, Soil Pollut 2302(230):1–17. https://doi.org/10.1007/S11270-019-4097-X
Noorani Khomeyrani SF, Azqhandi Azqhandi MH, Ghalami-Choobar B (2021) Rapid and efficient ultrasonic assisted adsorption of PNP onto LDH-GO-CNTs: ANFIS, GRNN and RSM modeling, optimization, isotherm, kinetic, and thermodynamic study. J Mol Liq 333:115917. https://doi.org/10.1016/J.MOLLIQ.2021.115917
Noroozi R, Al-Musawi TJ, Kazemian H et al (2018) Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. J Water Process Eng 21:44–51. https://doi.org/10.1016/J.JWPE.2017.11.011
Onu CE, Nwabanne JT, Ohale PE, Asadu CO (2021) Comparative analysis of RSM, ANN and ANFIS and the mechanistic modeling in eriochrome black-T dye adsorption using modified clay. South African J Chem Eng 36:24–42. https://doi.org/10.1016/J.SAJCE.2020.12.003
Pan D, Fan F, Wang Y et al (2017) Retention of Eu(III) in muscovite environment: batch and spectroscopic studies. Chem Eng J 330:559–565. https://doi.org/10.1016/J.CEJ.2017.07.184
Qiu HJ, Liu L, Wang Y (2016) Template-directed fabrication of 3D graphene-based composite and their electrochemical energy-related applications. Sci Bull 61:443–450. https://doi.org/10.1007/S11434-016-1024-Z
Raut DR, Mohapatra PK, Choudhary MK, Nayak SK (2013) Evaluation of two calix-crown-6 ligands for the recovery of radio cesium from nuclear waste solutions: solvent extraction and liquid membrane studies. J Memb Sci 429:197–205. https://doi.org/10.1016/J.MEMSCI.2012.11.045
Sadeghizadeh A, Ebrahimi F, Heydari M et al (2019) Adsorptive removal of Pb(II) by means of hydroxyapatite/chitosan nanocomposite hybrid nanoadsorbent: ANFIS modeling and experimental study. J Environ Manage 232:342–353. https://doi.org/10.1016/J.JENVMAN.2018.11.047
Shami RB, Shojaei V, Khoshdast H (2019) Efficient cadmium removal from aqueous solutions using a sample coal waste activated by rhamnolipid biosurfactant. J Environ Manage 231:1182–1192. https://doi.org/10.1016/J.JENVMAN.2018.03.126
Sun Y, Shao D, Chen C et al (2013) Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ Sci Technol 47:9904–9910. https://doi.org/10.1021/es401174n
Taka AL, Fosso-Kankeu E, Pillay K (2018) Mbianda XY (2018) Removal of cobalt and lead ions from wastewater samples using an insoluble nanosponge biopolymer composite: adsorption isotherm, kinetic, thermodynamic, and regeneration studies. Environ Sci Pollut Res 2522(25):21752–21767. https://doi.org/10.1007/S11356-018-2055-6
Tayyebi A, Outokesh M, Moradi S, Doram A (2015) Synthesis and characterization of ultrasound assisted “graphene oxide–magnetite” hybrid, and investigation of its adsorption properties for Sr(II) and Co(II) ions. Appl Surf Sci 353:350–362. https://doi.org/10.1016/J.APSUSC.2015.06.087
Thomas MF, Johnson CE, Dickson DPE, Berry FJ (eds) (1986) Mössbauer Spectroscopy. Cambridge University Press, Cambridge
Vanhoudt N, Van Ginneken P, Nauts R, Van Hees M (2018) Potential of four aquatic plant species to remove 60Co from contaminated water under changing experimental conditions. Environ Sci Pollut Res Int 25:27187–27195. https://doi.org/10.1007/S11356-018-2759-7
Vivas EL, Cho K (2021) Efficient adsorptive removal of Cobalt(II) ions from water by dicalcium phosphate dihydrate. J Environ Manage 283:111990. https://doi.org/10.1016/J.JENVMAN.2021.111990
Wei J, Aly Aboud MF, Shakir I et al (2020) Graphene oxide-supported organo-montmorillonite composites for the removal of Pb(II), Cd(II), and As(V) contaminants from water. ACS Appl Nano Mater 3:806–813. https://doi.org/10.1021/acsanm.9b02311
Wu D, Zhu C, Chen Y et al (2012) Preparation, characterization and adsorptive study of rare earth ions using magnetic GMZ bentonite. Appl Clay Sci 62–63:87–93. https://doi.org/10.1016/J.CLAY.2012.04.015
Wu H, Lin S, Cheng X et al (2020) Comparative study of strontium adsorption on muscovite, biotite and phlogopite. J Environ Radioact 225:106446. https://doi.org/10.1016/j.jenvrad.2020.106446
Wu H, Ma B, Chen J et al (2021) Insight into the adsorption of europium(III) on muscovite and phlogopite: effects of pH, electrolytes, humic substances and mica structures. Chemosphere 282:131087. https://doi.org/10.1016/J.CHEMOSPHERE.2021.131087
Wu W, Jiang CZ, Roy VAL (2016) Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 8:19421–19474. https://doi.org/10.1039/C6NR07542H
Xu W, Chen Y, Zhang W, Li B (2019) Fabrication of graphene oxide/bentonite composites with excellent adsorption performances for toluidine blue removal from aqueous solution. Adv Powder Technol 30:493–501. https://doi.org/10.1016/j.apt.2018.11.028
Yang S, Okada N, Nagatsu M (2016) The highly effective removal of Cs+ by low turbidity chitosan-grafted magnetic bentonite. J Hazard Mater 301:8–16. https://doi.org/10.1016/J.JHAZMAT.2015.08.033
Ye S, Yang Z, Xu J et al (2019) Clay–graphene oxide liquid crystals and their aerogels: synthesis, characterization and properties. R Soc Open Sci 6:181439. https://doi.org/10.1098/RSOS.181439
Yuan P, Fan M, Yang D et al (2009) Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr(VI)] from aqueous solutions. J Hazard Mater 166:821–829. https://doi.org/10.1016/J.JHAZMAT.2008.11.083
Zhang W, An Y, Li S et al (2020a) Enhanced heavy metal removal from an aqueous environment using an eco-friendly and sustainable adsorbent. Sci Reports 10:1–19. https://doi.org/10.1038/s41598-020-73570-7
Zhang H, Dong Y, He H et al (2020b) Sorption of cesium on Tamusu clay in synthetic groundwater with high ionic strength. Radiochim Acta 108:287–296. https://doi.org/10.1515/ract-2019-3161
Zhang X, Gu P, Liu Y (2019) Decontamination of radioactive wastewater: state of the art and challenges forward. Chemosphere 215:543–553. https://doi.org/10.1016/J.CHEMOSPHERE.2018.10.029
Zhao Y, Liu Y, Zhang X, Liao W (2021) Environmental transformation of graphene oxide in the aquatic environment. Chemosphere 262:127885. https://doi.org/10.1016/j.chemosphere.2020.127885
Zheng X, Dou J, Yuan J et al (2017) Removal of Cs+ from water and soil by ammonium-pillared montmorillonite/Fe3O4 composite. J Environ Sci 56:12–24. https://doi.org/10.1016/J.JES.2016.08.019
Zhidkin AP, Shamshurina EN, Golosov VN et al (2020) Detailed study of post-Chernobyl Cs-137 redistribution in the soils of a small agricultural catchment (Tula region, Russia). J Environ Radioact 223–224:106386. https://doi.org/10.1016/j.jenvrad.2020.106386
Author information
Authors and Affiliations
Contributions
Raman Novikau designed the composite, carried out important adsorption experiments, conducted data processing, and wrote the manuscript of the paper. Galina Lujanienė led the research and edited the manuscript. Vidas Pakštas, Martynas Talaikis, Kęstutis Mažeika, Audrius Drabavičius, and Arnas Naujokaitis did the characterisation of the composite. Sergej Šemčuk technical help. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
We affirm that this manuscript is an original work and that this work has neither been accepted nor submitted simultaneously to other journals.
Consent to participate
All listed authors have approved the manuscript.
Consent for publication
This manuscript has been approved by all authors for publication in Environmental Science and Pollution Research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Novikau, R., Lujanienė, G., Pakštas, V. et al. Adsorption of caesium and cobalt ions on the muscovite mica clay-graphene oxide-γ-Fe2O3-Fe3O4 composite. Environ Sci Pollut Res 29, 74933–74950 (2022). https://doi.org/10.1007/s11356-022-21078-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21078-0