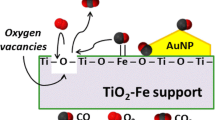
Abstract
The present work highlights the versatility of a TiO2-Al2O3 mixed oxide bearing highly dispersed gold nanoparticles that was applied in the CO oxidation reaction at room temperature. The TiO2, Al2O3, and TiO2-Al2O3 supports were synthesized by the sol–gel method, while gold nanoparticles were added by the deposition–precipitation with urea method using a theoretical Au loading of 2 wt.%. A promotional effect of the TiO2-Al2O3 support on the activity of gold catalysts with respect to TiO2 and Al2O3 was observed; Au/TiO2-Al2O3 showed outstanding CO oxidation, being active from 0 °C and stable throughout a 24-h test. As for the alumina content (5, 10, and 15 wt.%) in TiO2, it improved the textural properties by retarding the crystal growth and anatase–rutile phase transformation of TiO2, suppressing the deposition of carbon on the catalyst surface and stabilizing the Au nanoparticles even at high temperatures. Gold was highly dispersed with nanoparticle sizes ranging from 1 to 2 nm when H2 was used to treat thermally the Au/TiO2-Al2O3, Au/TiO2, and Au/Al2O3 materials. In addition, the XPS technique helped elicit that Au0 and Au1+ boosted their interaction with the TiO2, Al2O3, and TiO2-Al2O3 supports by means of charge transfer, which resulted in outstanding CO oxidation activity from 0 °C. Likewise, the key factors that control the peculiar catalytic performance in the CO oxidation reaction are discussed, which represents a step forward in the versatility behavior of gold catalysts supported on mixed oxide catalysts.










Similar content being viewed by others
Data availability
Not applicable.
References
Albonetti S, Bonelli R, EpoupaMengou J, Femoni C, Tiozzo C, Zacchini S, Trifiro F (2008) Gold/iron carbonyl clusters as precursors for TiO2 supported catalysts. Catal Today 137:483–488
Avgouropoulos G, Papavasiliou J, Ioannides T (2008) PROX reaction over CuO–CeO2 catalyst with reformate gas containing methanol. Catal Commun 9:1656–1660
Bakhshayesh AM, Mohammadi MR, Fray DJ (2012) Controlling electron transport rate and recombination process of TiO2 dye-sensitized solar cells by design of double-layer films with different arrangement modes. Electrochim Acta 78:384–391
Boaro M, Vicario M, Llorca J, de Leitenburg C, Dolcetti G, Trovarelli A (2009) A comparative study of water gas shift reaction over gold and platinum supported on ZrO2 and CeO2–ZrO2. App Catal B 88:272–282
Bokhimi X, Boldu JL, Muñoz E, Novaro O, Lopez T, Hernandez J, Gomez R, Garcia-Ruiz A (1999) Structure and composition of the nanocrystalline phases in a MgO-TiO2 system prepared via sol−gel technique. Chem Mater 11:2716–2721
Bouslama M, Amamra MC, Jia Z, Ben Amar MK, Chhor O, Brinza M, AbderrabbaVignes JL, Kanaev A (2012) Nanoparticulate TiO2–Al2O3 photocatalytic media: effect of particle size and polymorphism on photocatalytic activity. ACS Catal 2(9):1884–1892
Brijaldo MH, Passos FB, Rojas HA et al (2014) Hydrogenation of m-dinitrobenzene over Pt supported catalysts on TiO2–Al2O3 binary oxides. Catal Lett 144:860–866
Camposeco R, Zanella R (2022) Activity boosting of gold nanoparticles supported on V2O5/TiO2 nanostructures for CO oxidation at low temperature. Catal Today 392–393:49–59
Camposeco R, Castillo S, Mejía-Centeno I, Navarrete J, Nava N (2015) Boosted surface acidity in TiO2 and Al2O3-TiO2 nanotubes as catalytic supports. Appl Surf Sci 356:115–123
Carlsson P, Osterlund L, Thormahlen P, Palmqvist A, Fridell E, Jansson J, Skoglundh MA (2004) Transient in situ FTIR and XANES study of CO oxidation over Pt/AlO catalysts. J Catal 226:422–434
Costello CK, Kung MC, Oh HS, Wang Y, Kung HH (2002) Nature of the active site for CO oxidation on highly active Au/γ-Al2O3. Appl Catal A 232(1–2):159–168
Casapu M, Fischer A, Gänzler AM, Popescu R, Crone M, Gerthsen D, Türk M, Grunwaldt JD (2016) Origin of the normal and inverse hysteresis behavior during CO oxidation over Pt/Al2O3. ACS Catal 7:343–355
Date M, Okumura M, Tsubota S, Haruta M (2004) Vital role of moisture in the catalytic activity of supported gold nanoparticles. Angew Chem Int Ed 43(16):2129–2132
Duan A, Li R, Jiang G, Gao J, Zhao Z, Wan G, Zhang D, Huang W, Chung KH (2009) Hydrodesulphurization performance of NiW/TiO2-Al2O3 catalyst for ultra clean diesel. Catal Today 140(3–4):187–191
Engel T, Ertl G (1979) Elementary steps in the catalytic oxidation of carbon monoxide on platinum metals. In: Advances in catalysis, vol 28. Academic Press, Cambridge, pp 1–78
Galindo I, de Los Reyes J (2007) Effect of alumina–titania supports on the activity of Pd, Pt and bimetallic Pd–Pt catalysts for hydrorefining applications. Fuel Process Technol 88(9):859–863
Gavrila D, Georgakab A, Loukopoulosb V et al (2006) On the mechanism of selective CO oxidation on nanosized Au/γ-Al2O3 catalysts. Gold Bull 39:192–199
Glez V, Castillo S, Morán-Pineda M, Zanella R, Gomez R (2009) Effect of TiO2 In2O3 and TiO2 Al2O3 sol-gel supports on the morphology of gold nanoparticles. J Nano Res 5:1–12
Gómez-Cortés A, Díaz G, Zanella R, Ramírez H, Santiago P, Saniger JM (2009) Au-Ir/TiO2 prepared by deposition precipitation with urea: improved activity and stability in CO oxidation. J Phys Chem C 113:9710–9720
Gualteros JAD, Garcia MAS, da Silva AGM et al (2019) Synthesis of highly dispersed gold nanoparticles on Al2O3, SiO2, and TiO2 for the solvent-free oxidation of benzyl alcohol under low metal loadings. J Mater Sci 54:238–251
Guan H, Lin J, Qiao B, Yang X, Li L, Miao S, Liu J, Wang A, Wang X, Zhang T (2016) Catalytically active Rh sub-nanoclusters on TiO2 for CO oxidation at cryogenic temperatures. Angew Chem Int 55:2820–2824
Grunwaldt JD, Maciejewski M, Becker OS, Fabrizioli P, Baiker A (1999) Comparative study of Au/TiO2 and Au/ZrO2 catalysts for low-temperature CO oxidation. J Catal 186:458–469
Haruta M, Kobayashi T, Sano H, Yamada N (1987) Novel gold catalysts for the oxidation of carbon-monoxide at a temperature far below 0 °C. Chem Lett 16:405–408
Hassanisaadi M, Bonjar GHS, Rahdar A, Pandey S, Hosseinipour A, Abdolshahi R (2021) Environmentally safe biosynthesis of gold nanoparticles using plant water extracts. Nanomaterials 11(8):2033
Huang W, Duan A, Zhao Z, Wan G, Jiang G, Dou T (2008) Ti-modified alumina supports prepared by sol–gel method used for deep HDS catalysts. Catal Today 131:314–321
Lakshmanan P, Park J, Park E (2014) Recent advances in preferential oxidation of CO in H2 over gold catalysts. Catal Surv Asia 18(2–3):75–88
Lopez T, Bosch P, Tzompantzi F, Gomez R, Navarrete J, Lopez-Salinas E, Llanos ME (2000) Effect of sulfation methods on TiO2–SiO2 sol–gel catalyst acidity. Appl Catal A 197:107–117
Ma Z, Dai S (2011) Development of novel supported gold catalysts: a materials perspective. Nano Res 4:3–32
Masoud N, Partsch T, de Jong KP (2019) Thermal stability of oxide-supported gold nanoparticles. Gold Bull 52:105–114
Mendialdua J, Casanova R, Barbaux Y (1995) XPS studies of V2O5, V6O13, VO2 and V2O3. J Electron Spectrosc Relat Phenom 71:249–261
Morán-Pineda M, Castillo S, Asomoza M, Gomez R (2002) Al2O3-TiO2 sol-gel mixed oxides as suitable supports for the reduction of NO by CO. React Kinet Catal Lett 76:75–81
Newton M (2017) Time resolved operando X-ray techniques in catalysis, a case study: CO oxidation by O2 over Pt surfaces and alumina supported Pt catalysts. Catalysts 7(2):58
Okumura M, Nakamura S, Tsubota S, Nakamura T, Azuma M, Haruta M (1998) Chemical vapor deposition of gold on Al2O3, SiO2, and TiO2 for the oxidation of CO and of H2. Catal Lett 51:53–58
Oliveira RL, Bitencourt IG, Passos FB (2013) Partial oxidation of methane to syngas on Rh/Al2O3 and Rh/Ce-ZrO2 catalysts. J Braz Chem Soc 24:68–75
Reddy BM, Rao KN, Reddy GK, Bharali PJ (2006) Characterization and catalytic activity of V2O5/Al2O3-TiO2 for selective oxidation of 4-methylanisole. Mol Catal A 253(1):44–51
Romero-Sarria F, Penkova A, Martinez LM, Centeno MA, Hadjiivanov K, Odriozola JA (2008) Role of water in the CO oxidation reaction on Au/CeO2: modification of the surface properties. Appl Catal B 84:119–124
Saavedra J, Pursell CJ, Chandler BD (2018) CO oxidation kinetics over Au/TiO2 and Au/Al2O3 catalysts: evidence for a common water-assisted mechanism. J Am Chem Soc 140(10):3712–3723
Salanov AN, Savchenko VI (1985) TD and AES studies of the interaction of oxygen with Rh(100). React Kinet Catal Lett 29:101–109
Salomons S, Votsmeier M, Hayes RE, Drochner A, Vogel H, Gieshof J (2006) CO and H2 oxidation on a platinum monolith diesel oxidation catalyst. Catal Today 117:491–497
Soares JMC, Morrall P, Crossley A, Harris P, Bowker M (2003) Catalytic and noncatalytic CO oxidation on Au/TiO2 catalysts. J Catal 219:17–24
Tavizón-Pozos, JA, Suárez-Toriello VA, de los Reyes JA, Guevara-Lara A, Pawelec B, Fierro JLG, Vrinat M, Geantet C (2016) Deep hydrodesulfurization of dibenzothiophenes over niw sulfide catalysts supported on sol-gel titania-alumina. Top Catal 59:241–251
Trautmann S, Baerns M (1994) Infrared spectroscopic studies of CO adsorption on rhodium supported by SiO2, Al2O3, and TiO2. J Catal 150:335–344
Tsai Y, Chao H, Lin H (2009) Low temperature carbon monoxide oxidation over gold nanoparticles supported on sodium titanate nanotubes. J of Mol Catal A Chem 298:115–124
Valange S, Védrine J (2018) General and prospective views on oxidation reactions in heterogeneous catalysis. Catalysts 8:483
Wenfu Y, Zhen M, Shannon M, Jian J, Edward H, Steven O, Sheng D (2008) Novel Au/TiO2/Al2O3·xH2O catalysts for CO oxidation. Catal Lett 21(3–4):209–218
Wu S, Han H, Tai Q, Zhang J, Xu S, Zhou C, Yang Y, Hu H, Chen B, Zhao X (2008) Improvement in dye-sensitized solar cells employing TiO2 electrodes coated with Al2O3 by reactive direct current magnetron sputtering. J Power Source 182(1):119–123
Yablonskii GS, Lazman MZ (1996) New correlations to analyze isothermal critical phenomena in heterogeneous catalysis reactions (“Critical simplification”, “hysteresis thermodynamics”). React Kinet Catal Lett 59:145–150
Yang J, Bai H, Tan X, Lian J (2006) IR and XPS investigation of visible-light photocatalysis-nitrogen-carbon-doped TiO2 film. App Surf Sci 253:1988–1994
Zanella R, Louis C (2005) Influence of the conditions of thermal treatments and of storage on the size of the gold particles in Au/TiO2 samples. Catal Today 107–108:768–777
Zanella R, Giorgio S, Shin CH, Henry CR, Louis C (2004) Characterization and reactivity in CO oxidation of gold nanoparticles supported on TiO2 prepared by deposition-precipitation with NaOH and urea. J Catal 222:357–367
Zhang ZJ, Yates T (2012) Band bending in semiconductors: chemical and physical consequences at surfaces and interfaces. Chem Rev 112:5520–5551
Acknowledgements
The authors want to thank the financial support provided by the Consejo Nacional de Ciencia y Tecnología (CONACYT) through the CB A1-S-18269 grant, Dirección General de Asuntos del Personal Académico-UNAM through the PAPIIT IN104022 grant.
Funding
Consejo Nacional de Ciencia y Tecnología (CONACYT) through the CB A1-S-18269 grant, Dirección General de Asuntos del Personal Académico-UNAM through the PAPIIT IN104022 grant.
Author information
Authors and Affiliations
Contributions
Roberto Camposeco: formal analysis, writing—original draft preparation, conceptualization, and validation; investigation; Rodolfo Zanella: formal analysis, investigation, writing—original draft preparation, supervision, and project administration.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Camposeco, R., Zanella, R. Catalytic behavior of gold nanoparticles supported on a TiO2-Al2O3 mixed oxide for CO oxidation at low temperature. Environ Sci Pollut Res 29, 76992–77006 (2022). https://doi.org/10.1007/s11356-022-21076-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21076-2