Abstract
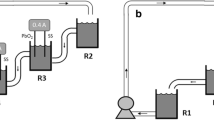
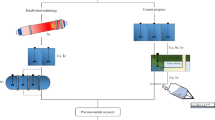
Electrochemical oxidation of trivalent chromium from leather tanning mud waste leachates (containing ca 6 g.L−1 Cr(III)) to its hexavalent form was carried out using a PbOx/Pb anode electrode in a prototype small (0.4 L) cylindrical batch electrochemical reactor. The PbOx/Pb anode was prepared by electrochemical anodization at constant current (75 mA cm−2 for 30 min) in a sulfuric acid solution and characterized by the cyclic voltammetry technique to investigate the effect of pH on the process. It was found that at pH = 3, Cr(III) oxidation prevails over the competing water oxidation-oxygen evolution reaction (OER), hence increasing the efficiency of the process. A detailed study of pH (0–3), current density (12–24 mA cm−2), and cell type (divided-undivided) effects on bulk electrolysis of Cr(III) leachates in the batch prototype reactor resulted in process optimization. At pH = 3, 12 mA cm−2 and a cathode inserted in a porous diaphragm envelope, nearly 70% conversion was achieved at a nearly 60% current efficiency, among the highest in the previously reported literature. The method (further optimized with an ion-selective membrane separator) could offer an attractive route for tannery Cr(III) conversion to Cr(VI) for reuse as an etchant or electroplating agent.
Graphical abstract









Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Blood PJ, Brown IJ, Sotiropoulos S (2004) Electrodeposition of lead dioxide on carbon substrates from a high internal phase emulsion (HIPE). J Appl Electrochem 34:1–7
Bykovsky NA, Kantor EA, Rahman PA, Puchkova LN, Fanakova NN (2020) Trivalent chromium wastewater treatment. IOP Conf Ser: Mater Sci Eng 775:012085. https://doi.org/10.1088/1757-899X/775/1/012085
Chen Z, Xie G, Pan Z, Zhou X, Lai W, Zheng L, Xu Y (2021) A novel Pb/PbO2 electrodes prepared by the method of thermal oxidation-electrochemical oxidation: characteristic and electrocatalytic oxidation performance. J Alloys Compd 851:156834. https://doi.org/10.1016/j.jallcom.2020.156834
Danilov FI, Velichenko AB (1993) Electrocatalytic activity of anodes in reference to Cr(IIΙ) oxidation reaction. Ektrochimico Acta 38:437-440.
Devilliers D, Dinh Thi MT, Mahé E, le Xuan Q (2003) Cr(III) oxidation with lead dioxide-based anodes. Electrochim Acta 48(28):4301–4309. https://doi.org/10.1016/j.electacta.2003.07.005
Fernandes A, Santos D, Pacheco MJ, Ciríaco L, Lopes A (2016) Electrochemical oxidation of humic acid and sanitary landfill leachate: Influence of anode material, chloride concentration and current density. Sci Total Environ 541:282–291. https://doi.org/10.1016/j.scitotenv.2015.09.052
Grace Pavithra K, Jaikumar V, Kumar PS, SundarRajan PS (2019) A review on cleaner strategies for chromium industrial wastewater: present research and future perspective. J Clean Prod 228:580–593. https://doi.org/10.1016/j.jclepro.2019.04.117
Kokkinos E, Banti A, Mintsouli I, Touni A, Sotiropoulos S, Zouboulis A (2021) Combination of thermal, hydrometallurgical and electrochemical tannery waste treatment for Cr(III) recovery. Appl Sci (Switzerland) 11(2):1–14. https://doi.org/10.3390/app11020532
Kokkinos E, Zouboulis AI (2020a) The chromium recovery and reuse from tanneries: a case study according to the principles of circular economy. In: Muthu S (ed) Leather and Footwear Sustainability, 1st edn. Springer, Singapore, p 123–157. https://doi.org/10.1007/978-981-15-6296-9_6
Kokkinos E, Zouboulis A (2020b) Hydrometallurgical recovery of Cr(III) from tannery waste: optimization and selectivity investigation. Water (Switzerland) 12:719–728. https://doi.org/10.3390/w12030719
Koppad VB (2014) IJERT performance evaluation of electrochemical oxidation system to treat domestic sewage performance evaluation of electrochemical oxidation system to treat domestic sewage. Int J Eng Res Technol 3:IJERTV3IS060929 www.ijert.org
Kuppusamy S, Jayaraman N, Jagannathan M, Kadarkarai M, Aruliah R (2017) Electrochemical decolorization and biodegradation of tannery effluent for reduction of chemical oxygen demand and hexavalent chromium. J Water Process Eng 20:22–28. https://doi.org/10.1016/j.jwpe.2017.09.008
Lateef H, Grimes SM, Chaudhary AJ, Goswami NC (2009) Opportunity to recycle chromium(VI) by in situ electro-oxidation. J Chem Technol Biotechnol 84(4):584–588. https://doi.org/10.1002/jctb.2084
Li X, Pletcher D, Walsh FC (2011) Electrodeposited lead dioxide coatings. Chem Soc Rev 40(7):3879–3894. https://doi.org/10.1039/c0cs00213e
Li J, Bai J, Huang K, Zhou B, Wang Y, Hu X (2014) Removal of trivalent chromium in the complex state of trivalent chromium passivation wastewater. Chem Eng J 236:59–65. https://doi.org/10.1016/j.cej.2013.09.084
Martínez-Huitle CA, Panizza M (2018) Electrochemical oxidation of organic pollutants for wastewater treatment. Curr Opin Electrochem 11:62–71. https://doi.org/10.1016/j.coelec.2018.07.010
Ouejhani A, Hellal F, Dachraoui M, Lallevé G, Fauvarque JF (2008) Application of Doehlert matrix to the study of electrochemical oxidation of Cr(III) to Cr(VI) in order to recover chromium from wastewater tanning baths. J Hazard Mater 157(2–3):423–431. https://doi.org/10.1016/j.jhazmat.2008.01.046
Panizza M, Martinez-Huitle CA (2013) Role of elce anodic oxidation of a real landfill leachate - comparison between Ti-Ru-Sn ternary oxide, PbO2 and boron-doped diamond anode. Chemosphere 90(4):1455–1460. https://doi.org/10.1016/j.chemosphere.2012.09.006
Selvakumar AM, Vimudha M, Lawrance I, Sundaramoorthy S, Ramanaiah B, Saravanan P (2019) Recovery and reuse of spent chrome tanning effluent from tannery using electro-oxidation technique. Desalin Water Treat 156:323–330. https://doi.org/10.5004/dwt.2019.23825
Touni A, Papaderakis A, Karfaridis D, Vourlias G, Sotiropoulos S (2019) Oxygen evolution reaction at IrO2/Ir(Ni) film electrodes prepared by galvanic replacement and anodization: effect of precursor Ni film thickness. Molecules 24:2095. https://doi.org/10.3390/molecules24112095
US EPA (1992), Test Method 7196A: Chromium, Hexavalent (Colorimetric).https://www.epa.gov/hw-sw846/sw-846-test-method-7196a-chromium-hexavalentcolorimetric. Accessed 1 Nov 2021.
Wang C, Yin L, Xu Z, Niu J, Hou LA (2017) Electrochemical degradation of enrofloxacin by lead dioxide anode: kinetics, mechanism and toxicity evaluation. Chem Eng J 326:911–920. https://doi.org/10.1016/j.cej.2017.06.038
Xu T, Nan F, Jiang X, Tang Y, Zeng Y, Zhang W, Shi B (2020) Effect of soil pH on the transport, fractionation, and oxidation of chromium(III). Ecotoxicol Environ Saf 195:110459. https://doi.org/10.1016/j.ecoenv.2020.110459
Xu T, Jiang X, Tang Y, Zeng Y, Zhang W, Shi B (2021) Oxidation of trivalent chromium induced by unsaturated oils: a pathway for hexavalent chromium formation in soil. J Hazard Mater 405:124699. https://doi.org/10.1016/j.jhazmat.2020.124699
Zhao X, Li C, Wang Y, Xu W (2018) Electrodeposited PbO2 thin films with different surface structure as positive plate in lead acid batteries. Int J Electrochem Sci 13(4):3745–3756. https://doi.org/10.20964/2018.04.52
Zhou B, Yu Z, Wei Q, Long HY, Xie Y, Wang Y (2016) Electrochemical oxidation of biological pretreated and membrane separated landfill leachate concentrates on boron doped diamond anode. Appl Surf Sci 377:406–415. https://doi.org/10.1016/j.apsusc.2016.03.045
Zhuo Q, Luo M, Guo Q, Yu G, Deng S, Xu Z, Yang B, Liang X (2016) Electrochemical oxidation of environmentally persistent perfluorooctane sulfonate by a novel lead dioxide anode. Electrochim Acta 213:358–367. https://doi.org/10.1016/j.electacta.2016.07.005
Zou J, Peng X, Li M, Xiong Y, Wang B, Dong F, Wang B (2017) Electrochemical oxidation of COD from real textile wastewaters: kinetic study and energy consumption. Chemosphere 171:332–338. https://doi.org/10.1016/j.chemosphere.2016.12.065
Acknowledgements
The authors thank Anna Mavridou and Olga Orfanou for making arrangements and carrying out SEM and XRD measurements.
Funding
This work was supported by the project “INVALOR: Research Infrastructure for Waste Valorization and Sustainable Management” (MIS 5002495) which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure,” funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund).
Author information
Authors and Affiliations
Contributions
Conceptualization: Sotiris Sotiropoulos; methodology: Sotiris Sotiropoulos, Angeliki Banti; data curation: Angeliki Banti, Orestis Grammenos; writing—original draft preparation: Sotiris Sotiropoulos, Angeliki Banti; writing—review and editing: Εvgenios Kokkinos, Anastasios Zouboulis, Sotiris Sotiropoulos; funding acquisition: Anastasios Zouboulis; investigation: Orestis Grammenos, Aikaterini Touni; supervision: Sotiris Sotiropoulos, Anastasios Zouboulis; visualization: Angeliki Banti, Εvgenios Kokkinos.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ioannis A. Katsoyiannis
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Banti, A., Grammenos, O., Kokkinos, Ε. et al. Electrochemical conversion of chromium from tannery effluents for potential reuse in industrial applications. Environ Sci Pollut Res 30, 8722–8731 (2023). https://doi.org/10.1007/s11356-022-19985-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19985-3