Abstract
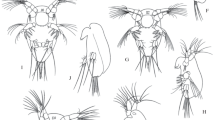
Description and morphological analysis of copepods inhabiting a water body with high arsenic concentrations (32.79 to 62.29 mg L−1) were performed to identify some effect on the development of individuals due to the arsenic concentrations. Detailed morphology of prosomal and urosomal appendages along the development of the specimens was considered. The results showed that the freshwater copepod Paracyclops novenarius Reid, 1987 inhabits this water body, and previously, it was recorded as Paracyclops chiltoni (Thomson GM, 1882) on this site. Moreover, this becomes the first record of P. novenarius in Mexico. Morphological analysis showed a normal and stable development along the different instars, different arsenic concentrations in the media, and different sampled dates between the analyzed specimens, suggesting that the high arsenic concentrations do not affect the morphology of P. novenarius, including all its development and adult instars, which differs from other copepods and other groups such as Cladocera and Rotifera, where morphological changes due to metals and metalloids have been observed but in low concentrations of these elements. The results of this study contribute to the existing reports of the genus Paracyclops (Claus 1893) in Mexico and could provide information for environmental impact assessments on aquatic systems.





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the article.
References
Alvarado-Flores JR (2014) Detection of the effect of endocrine disruptors and metals on the reproduction of freshwater rotifers. PhD Thesis. Autonomous University of Aguascalientes. Mexico. 147 pp. (In Spanish)
Alvarado-Flores JR, Rico-Martínez A, Adabache-Ortíz S-B (2015) Morphological alterations in the freshwater rotifer Brachionus calyciflorus Pallas 1766 (Rotifera: Monogononta) caused by vinclozolin chronic exposure. Ecotoxicology 24:915–925
Araujo GS, Pavlaki MD, Soares AMVM, Abessa DMS, Loureiro S (2019) Bioaccumulation and morphological traits in a multi-generation test with two Daphnia species exposed to lead. Chemosphere 219:636–644. https://doi.org/10.1016/j.chemosphere.2018.12.049
Armienta MA, Amat PD, Larios T, López DL (2008) Central America and Mexico. In: Bundschuh J, Pérez-Carrera A, Litter M (eds). Distribution of arsenic in the Iberian and Ibero-American regions. ISBN: 13–978–84–96023–61–1 (In Spanish)
Arnold WR, Diamond RL, Smith DS (2010) The effects of salinity, pH, and dissolved organic matter on acute copper toxicity to the rotifer, Brachionus plicatilis (“L” strain). Arch Environ Contam Toxicol 2:225–234. https://doi.org/10.1007/s00244-010-9467-8
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6(4):189–213
Barrera RR, Chavarría GJ, Morales FJ (2010) Malignant mesothelioma: clinical and pathological features from 247 cases. Rev Chil Enf Respir 26:134–140. https://doi.org/10.4067/S0717-73482010000300003
Borgmann U, Couillard Y, Doyle P, Dixon DG (2005) Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environ Toxicol Chem 24(3):641–652. https://doi.org/10.1897/04-177r.1
Brandorff GO (2012) Distribution of some Calanoida (Crustacea: Copepoda) from the Yucatán Peninsula, Belize and Guatemala. Rev Biol Trop 60:187–202
Bundschuh J, Pérez-Carrera A, Litter M (2008) Introduction: Distribution of arsenic in the Iberian and Ibero-American regions. In: Bundschuh J, Pérez-Carrera A, Litter M (eds). Distribution of arsenic in the Iberian and Ibero-American regions. ISBN: 13–978–84–96023–61–1 (In Spanish)
Calabrese EJ (2008) Converging concepts: adaptative response, preconditioning and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res Rev 7:8–20. https://doi.org/10.1016/j.arr.2007.07.001
Castellano-Alvarado L, Enriquez JL, Barron D (1960) Asbestosis. Journal of Public Health of Mexico 2:557–566 (In Spanish)
Caumette G, Koch I, Estrada E, Reimer KJ (2011) Arsenic speciation in plankton organisms from contaminated lakes: transformations at the base of the freshwater food chain. Environ Sci Technol 45:9917–9923. https://doi.org/10.1021/es2025092
Caussy D, Priest ND (2008) Introduction to arsenic contamination and health risk assessment with special reference to Bangladesh. In: Garelick H, Jones H (eds) Reviews of environmental contamination and toxicology. Volume 197. ISBN: 978–0–387–79283–5
CEFIM (2016) Monographs of the municipalities of Mexico: Matehuala, San Luis Potosi. http://cefimslp.gob.mx/monografias_municipales/2012/venado/files/venado.12.pdf. Accessed 26 May 2021 (in Spanish)
Cervantes-Martínez A, Elías-Gutiérrez ME, Gutiérrez-Aguirre MA, Kotov AA (2005) Ecological remarks on Mastigodiaptomus nesus Bowman, 1986 (Copepoda: Calanoida) in a Mexican karstic sinkhole. Hydrobiologia 542:95–102. https://doi.org/10.1007/s10750-004-2488-4
Cervantes-Martínez A, Gutiérrez-Aguirre MA (2015) Physicochemistry and zooplankton of two karstic sinkholes in the Yucatan Peninsula, Mexico. J Limnol 74:382–393. https://doi.org/10.4081/jlimnol.2014.976
Chen CY, Sillett KB, Folt CL, Whittemore SL, Barchowsky A (1999) Molecular and demographic measures of arsenic stress in Daphnia pulex. Hydrobiologia 401:229–238. https://doi.org/10.1023/A:1003738427354
Collazos-Santos MF (2014). Definition of discharge objectives in Buenaventura Bay development phase I. Master’s theses. Autonomous University of Occident, Cali, Colombia. https://red.uao.edu.co/bitstream/handle/10614/5885/T03915.pdf?sequence=1&isAllowed=y. Accessed 15 June 2021 (In Spanish)
Dahms HU, Fernando CH (1992) Naupliar development of Mesocyclops aequatorialis similis and Thermocyclops consimilis (Copepoda: Cyclopoida) from Lake Awasa, a tropical rift valley lake in Ethiopia. Can J Zool 70:2283–2297. https://doi.org/10.1139/z92-306
Dahms HU, Won EJ, Kim HS, Han J, Park HG, Souissi S, Raisuddin S, Lee JS (2016) Potential of the small cyclopoid copepod Paracyclopina nana as an invertebrate model for ecotoxicity testing. Aquat Toxicol 180:282–294. https://doi.org/10.1016/j.aquatox.2016.10.013
DOF (1994) NOM-117-SSA1–1994 (Mexican Official Norm). Secretariat of Health, Mexico. http://www.ordenjuridico.gob.mx/Documentos/Federal/wo69541.pdf. Accessed 2 June 2021 (In Spanish)
DOF (1998) NOM-001-SEMARNAT- 1996 (Mexican Official Norm). Secretariat of Health, Mexico. https://www.profepa.gob.mx/innovaportal/file/3290/1/nom-001-semarnat-1996.pdf. Accessed 2 June 2021 (In Spanish)
Elkhodary GM, Elsayed HS (2011) Effect of cadmium and copper on the population growth and morphology of Branchionus plicatilis (Rotifera). Egypt J Exp Biol (zool) 7(2):323–328
Ferrari FD (2000) Patterns of setal numbers conserved during early development of swimming legs of Copepoda (Crustacea). Hydrobiologia 417:81–90. https://doi.org/10.1023/A:1003895004611
Gagneten AM, Paggi JC (2009) Effects of heavy metal contamination (Cr, Cu, Pb, Cd) and eutrophication on zooplankton in the lower basin of the Salado River (Argentina). Water Air Soil Pollut 198:317–334. https://doi.org/10.1007/s11270-008-9848-z
Galassi DMP, Huys R, Reid JW (2009) Diversity, ecology and evolution of groundwater copepods. Freshw Biol 54:691–708. https://doi.org/10.1111/j.1365-2427.2009.02185.x
Gama-Flores JL, Castellanos-Paez ME, Sarma SS, Nandini S (2007) Effect of pulsed exposure to heavy metals (copper and cadmium) on some population variables of Brachionus calyciflorus Pallas (Rotifera: Brachionidae: Monogononta). Hydrobiologia 593:201–208. https://doi.org/10.1007/s10750-007-9042-0
Gaviria S (1994) The free-living copepods (Arthropoda, Crustacea) of the continental waters of Colombia. Rev Acad Colomb Cienc 19:361–385 (In Spanish)
Gaviria S, Aranguren N (2007) Free-living species of the Copepoda (Arthropoda, Crustacea) subclass of the Colombian continental waters. Biota Colombiana 8:53–68 (In Spanish)
Gerten D, Adrian R (2002) Species-specific changes in the phenology and peak abundance of freshwater copepods in response to warm summers. Freshw Biol 47:2163–2173. https://doi.org/10.1046/j.1365-2427.2002.00970.x
Gómez-Márquez JL, Peña-Mendoza B, Guzmán-Santiago JL, Gallardo-Pineda V (2013) Zooplankton composition, abundance and water quality in a microreservoir at Morelos State. Hidrobiológica 23:227–240 (In Spanish)
González EJ, Matos ML, Peñaherrera C, Merayo S (2011) Zooplankton abundance, biomass and trophic state in some Venezuelan reservoirs. In Atazadeh E (ed.) Biomass and Remote Sensing of Biomass. ISBN: 978–953–51–6038–0
Gutiérrez MF, Gagneten AM (2011) Effects of metals on freshwater microcrustaceans. Methodological advances and potentiality of cladocerans and copepods as test organisms. Revista Peruana de Biología 18:389–396. https://doi.org/10.15381/rpb.v18i3.460 (In Spanish)
Gutiérrez MF, Gagneten AM, Paggi JC (2010) Copper and chromium alter life cycle variables and the equiproportional development of the freshwater copepod Notodiaptomus conifer (SARS). Water Air Soil Pollut 213:275–286. https://doi.org/10.1007/s11270-010-0383-3
Gutierrez MF, Paggi JC, Gagneten AM (2012) Microcrustaceans escape behavior as an early bioindicator of copper, chromium and endosulfan toxicity. Ecotoxicology 21:428–438. https://doi.org/10.1007/s10646-011-0803-1
Hall LW Jr, Anderson RD, Lewis BL, Arnold WR (2008) The influence of salinity and dissolved organic carbon on the toxicity of copper to the estuarine copepod, Eurytemora affinis. Arch Environ Contam Toxicol 54:44–56. https://doi.org/10.1007/s00244-007-9010-8
Hirst AG, Kiørboe T (2014) Macroevolutionary patterns of sexual size dimorphism in copepods. Proc R Soc B 28:20140739. https://doi.org/10.1098/rspb.2014.0739
Hose GC, Symington K, Lott MJ, Lategan MJ (2016) The toxicity of arsenic (III), chromium (VI) and zinc to groundwater copepods. Environ Sci Pollut Res 23(18):18704–18713. https://doi.org/10.1007/s11356-016-7046-x
Huys R, Boxshall GA (1991) Copepod evolution. The Ray Society, London
Hwang DS, Lee KW, Han J, Park HG, Lee J, Lee YM, Lee JS (2010) Molecular characterization and expression of vitellogenin (Vg) genes from the cyclopoid copepod, Paracyclopina nana exposed to heavy metals. Comp Biochem Physiol C 151:360–368. https://doi.org/10.1016/j.cbpc.2009.12.010
INEGI (2002) Synthesis of geographic information of San Luis Potosí. Institute of Statistics, Geography and informatics of Mexico. https://www.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvine gi/productos/historicos/2104/702825224240/702825224240_2.pdf. Accessed 16 May 2021
Karaytug S, Boxshall GA (1998) Partial revision of Paracyclops Claus, 1893 (Copepoda, Cyclopoida, Cyclopidae) with descriptions of four new species. Bull Nat Hist Mus Lond (zool) 64:111–205
Karaytug S, Boxshall GA (1998) The Paracyclops fimbriatus-complex (Copepoda, Cyclopoida): a revision. Zoosystema 20:563–602
Karaytug S, Boxshall GA (1999) Antennules of the male of Paracyclops (Copepoda): functional significance and their importance in systematics. J Crustac Biol 19:371–379. https://doi.org/10.1163/193724099X00187
Karaytug S, Defaye D, Boxshall GA (1998) Two new species of Paracyclops (Copepoda: Cyclopoida, Cyclopidae) from Africa. Hydrobiologia 382:119–136. https://doi.org/10.1023/A:1003473215548
Krupa EG (2005) Population densities, sex ratios of adults, and occurrence of malformations in three species of cyclopoid copepods in waterbodies with different degrees of eutrophy and toxic pollution. J Mar Sci Technol 13:226–237
Krupa EG, Barinova S, Romanova S, Aubakirova M, Ainabaeva N (2020) Planktonic invertebrates in the assessment of long-term change in water quality of the Sorbulak wastewater disposal system (Kazakhstan). Water 12:3409. https://doi.org/10.3390/w12123409
Krupa EG (2007) Structural characteristics of zooplankton of the Shardarinskoe reservoir and their use in water quality assessment. Water Resour 34:712–717
Lin KY, Sastri AR, Gong GC, Hsieh CH (2013) Copepod community growth rates in relation to body size, temperature, and food availability in the East China Sea: a test of metabolic theory of ecology. Biogeosciences 10:1877–1892. https://doi.org/10.5194/bg-10-1877-2013
Martínez-Villegas N, Briones-Gallardo R, Ramos-Leal JA, Avalos-Borja M, Castañon-Sandoval AD, Razo-Flores E, Villalobos M (2013) Arsenic mobility controlled by solid calcium arsenates: a case study in Mexico showcasing a potentially widespread environmental problem. Environ Pollut 176:114–122. https://doi.org/10.1016/j.envpol.2012.12.025
Melo PAMC, Neumann-Leitão S, Zanardi-Lamardo E, Melo-Júnior M (2021) Morphological abnormalities in Acartialilljeborgii Giesbrecht (1889) (Copepoda, Calanoida) in a tropical estuary under industrial development An Acad Bras Ciênc 93 https://doi.org/10.1590/0001-3765202120190231
Melo RRR, Coelho PN, Santos-Wisniewski MJ, Wisniewski C, Magalhães CS (2017) Morphological abnormalities in cladocerans related to eutrophication of a tropical reservoir. J Limnol 76:94–102. https://doi.org/10.4081/jlimnol.2016.1395
Mendoza-Chávez YJ, Uc-Castillo JL, Cervantes-Martínez A, Gutiérrez-Aguirre MA, Castillo-Michel H, Loredo-Portales R, SenGupta B, Martínez-Villegas N (2021) Paracyclops chiltoni inhabiting water highly contaminated with arsenic: water chemistry, population structure, and arsenic distribution within the organism. Environ Pollut 284:117155. https://doi.org/10.1016/j.envpol.2021.117155
Mercado-Salas N, Suárez-Morales E (2009) A new species and illustrated records of Paracyclops Claus, 1893 (copepoda: Cyclopoida: cyclopinae) from Mexico. J Nat Hist 43:2789–2808. https://doi.org/10.1080/00222930903108462
Mercado-Salas NF, Suárez-Morales E (2012) Morphology, diversity, and distribution of the Cyclopoida (Copepoda) from arid areas of central-north. Mexico. II Eucyclopinae and Biogeographic Analysis Hidrobiológica 22:99–124 (In Spanish)
Mitsuka PM, Henry R (2002) The fate of copepod populations in the Paranapanema River (São Paulo, Brazil), downstream from the Jurumirim dam. Braz Arch Biol Technol 45:479–490. https://doi.org/10.1590/S1516-89132002000600012
Mohammed EH, Wang G, Jiang J (2010) The effects of nickel on the reproductive ability of three different marine copepods. Ecotoxicology 19:911–916. https://doi.org/10.1007/s10646-010-0471-6
Navarro-Espinoza S, Angulo-Molina A, Meza-Figueroa D, López-Cervantes G, Meza-Montenegro M, Armienta A, Soto-Puebla D, Silva-Campa E, Burgara-Estrella A, Álvarez-Bajo O, Pedroza-Montero M (2021) Effects of untreated drinking water at three indigenous Yaqui towns in Mexico: insights from a murine model. Int J Environ Res Public Health 18(2):805. https://doi.org/10.3390/ijerph18020805
Pérez E, Hoang TC (2017) Chronic toxicity of binary-metal mixtures of cadmium and zinc to Daphnia magna. Environ Toxicol Chem 36:2739–2749. https://doi.org/10.1002/etc.3830
Pérez-Yañez D, Soriano-Martínez DR, Damian-Ku ME, Cejudo-Espinosa E, Alvarado-Flores J (2019) Cadmium and morphological alterations in the rotifer Philodina cf. roseola (Bdelloidea: Philodinidae) and the worm Aeolosoma hemprichi (Annelida: Aeolosomatidae). Rev Biol Trop 67:1406–1417
Plath K, Boersma M (2001) Mineral limitation of zooplankton: stoichiometric constraints and optimal foraging. Ecology 82:1260–1269. https://doi.org/10.1890/0012-9658(2001)082[1260:MLOZSC]2.0.CO;2
Rampelotto PH (2013) Extremophiles and extreme environments. Life 3:482–485. https://doi.org/10.3390/life3030482
Ravenscroft P, Brammer H, Richards K (2009) Arsenic pollution: a global synthesis. Singapore.
Razo I, Carrizales L, Castro J, Díaz-Barriga F, Monroy M (2004) Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water Air Soil Pollut 152:129–152. https://doi.org/10.1023/B:WATE.0000015350.14520.c1
Reid JW (1987) Some cyclopoid and harpacticoid copepods from Colombia, including descriptions of three new species. PROC BIOL SOC WASH 100:262–271
Ruíz-Huerta EA, de la Garza VA, Gómez-Bernal JM, Castillo F, Avalos-Borja M, SenGupta B, Martínez-Villegas N (2017) Arsenic contamination in irrigation water, agricultural soil and maize crop from an abandoned smelter site in Matehuala, Mexico. J Hazard Mater 339:330–339. https://doi.org/10.1016/j.jhazmat.2017.06.041
Sarma SSS, Osnaya-Espinosa LR, Aguilar-Acosta CR, Nandini S (2011) Seasonal variations in zooplankton abundances in the Iturbide reservoir (Isidro Fabela, State of Mexico, Mexico). J Environ Biol 32:473
Schubauer-Berigan MK, Dierkes JR, Monson PD, Ankley GT (1993) pH-dependent toxicity of Cd, Cu, Ni, Pb and Zn to Ceriodaphnia dubia, Pimephales promelas, Hyalella azteca and Lumbriculus variegatus. Environ Toxicol Chem 2:1261–1266. https://doi.org/10.1002/etc.5620120715
Sobrino-Figueroa A, Álvarez-Hernandez S, Álvarez Silva C (2020) Evaluation of the freshwater copepod Acanthocyclops americanus (Marsh, 1983) (Cyclopidae) response to Cd, Cr, Cu, Hg, Mn, Ni and Pb[J]. AIMS Environmental Science 7(6):449–463. https://doi.org/10.3934/environsci.2020029
Suárez-Morales E (2020) Diversity and distribution of the copepods (Cyclopoida) of the arid zones of North-Central Mexico. National Commission for the Knowledge and Use of Biodiversity. https://doi.org/10.15468/vbhfeb. Accessed 23 May 2021 (In Spanish)
Suárez-Morales E, Gutiérrez-Aguirre MA, Gómez S, Perbiche-Neves G, Previatelli D, dos Santos-Silva EN, da Rocha CEF, Mercado-Salas NF, Marques TM, Cruz Quintana Y, Santana Piñeros AM (2020). Class Copepoda. In: Damborenea C, Damborenea DC, Rogers, Thorp JH (eds.) Keys to Neotropical and Antarctic fauna, Thorp and Covich’s freshwater, invertebrates. Volume V. Fourth Edition. ISBN: 978–0–12–804225–0
Suárez-Morales E, Wyngaard G, Gutiérrez-Aguirre MA, Constanzo J (2007) Life history traits of Mesocyclops thermocyclopoides Harada, 1931 (Copepoda, Cyclopoida) with observations on naupliar morphology. Crustaceana 80:1205–1222. https://doi.org/10.1163/156854007782321146
Villagran DM, Fernández-Severini MD, Biancalana F, Spetter CV, Fernández EM, Marcovecchio JE (2019) Bioaccumulation of heavy metals in mesozooplankton from a human-impacted south western Atlantic estuary (Argentina). J Mar Res 77:217–241. https://doi.org/10.1357/002224019826887362
Williamson CE, Reid JW (2001) Copepoda. In Thorp JH, Covich AP (eds.) Ecology and classification of North American freshwater invertebrates. Academic, San Diego, USA.
Wong CK, Pak AP (2004) Acute and subchronic toxicity of the heavy metals copper, chromium, nickel, and zinc, individually and in mixture, to freshwater copepod Mesocyclos pehpeiensis. Bull. Environ. Contam. Toxicol 73: 190–196. https://doi.org/10.10007/s00128-004-0412-2
Xue YH, Yang XX, Zhang G, Xi YL (2017) Morphological differentiation of Brachionus calyciflorus caused by predation and coal ash pollution. Sci Rep 7:15779. https://doi.org/10.1038/s41598-017-16192-w
Zou E (2010) Aquatic invertebrate endocrine disruption Encyclopedia of Animal Behavior 112–123 https://doi.org/10.1016/B978-0-08-045337-8.00266-7
Acknowledgements
The authors thank Laboratory of Limnology and Tropical Ecology of University of Quintana Roo, Cozumel; PhD. Nadia Valentina Martínez-Villegas and PhD. Yadira Jazmín Mendoza-Chávez for the facilities and support in fieldwork, and National Laboratory of Agricultural, Medical and Environmental Biotechnology (LANBAMA-IPICYT) for the determination of arsenic in water samples. Facilities to use the Scanning Microscopy JEOL SM-6010 were provided by El Colegio de la Frontera Sur (ECOSUR, Chetumal). José Ángel Cohuó Colli kindly allowed us to review ECOCH-Z specimens. Sarahi Jaime helped us in the elaboration of some figures presented in this work.
Funding
Scholarship Granted for Unique Support (CH-22478 ) from the Potosino Institute of Scientific and Technological Research A.C. (IPICYT). University of Quintana Roo, Cozumel.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by JLUC, ACM, and MAGA. The first draft of the manuscript was written by JLUC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We collected from several freshwater ecosystems in Mexico. However, Mexican laws do not protect Zooplankton; thus, no specific permits for this type of field study are needed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uc-Castillo, J.L., Cervantes-Martínez, A. & Gutiérrez-Aguirre, M.A. Evaluation of arsenic effects on Paracyclops novenarius Reid, 1987: a cyclopoid copepod in central-north of Mexico. Environ Sci Pollut Res 29, 61674–61684 (2022). https://doi.org/10.1007/s11356-022-18959-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18959-9