Abstract
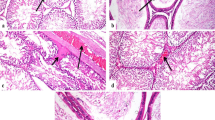
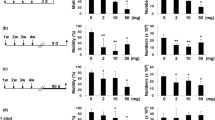
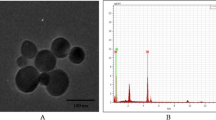
Silica nanoparticles (SiNPs), one of the most produced nanoparticles (NPs) in the world, are used in all aspects of life. The increased application of SiNPs, especially in medicine, has raised considerable concern regarding their toxicological impact. Previous studies have shown that SiNPs can pass through the reproductive barrier and cause reproductive organ dysfunction by destroying Sertoli cells, Leydig cells, and germ cells. However, little is known about the mechanism of SiNPs-induced reproductive toxicity. In the present study, 5-week-old male mice were intraperitoneally administered SiNPs per day for 1 week at a dose of 0.2 mg per mouse. The results showed that SiNPs could cause damage to the structure of the testis and the epididymis and change the reproductive organ coefficients, leading to decreases of 56.1% and 55.3% in the rates of sperm concentration and motility and an increase of 168.8% in the rate of sperm abnormality. Moreover, the serum testosterone level obviously decreased from 18.77 to 5.23 µg/ml after exposure, and the transcription statuses of some key genes involved in the synthesis and transport of testosterone in the testis were also affected. Additional experiments showed that SiNPs exposure during puberty induced oxidative stress and an inflammatory response, as shown by the changed activity of superoxide dismutase (SOD), increased contents of malondialdehyde (MDA), and excess expression of proinflammatory factors, including TNF-α and IL-1β. Furthermore, the administration of SiNPs caused DNA damage and cell apoptosis, which were presented by the increased apoptotic cells in the sections of testis and epididymis and activation of the TNF-α/TNFR I-mediated pro-apoptotic pathway. In conclusion, these results indicate that SiNPs exposure during puberty significantly damaged the structure and function of the testis and epididymis by inducing oxidative stress and cell apoptosis. This study provides novel insight into SiNPs-induced reproductive toxicity during puberty, which warrants a more careful assessment of SiNPs before their application in juvenile supplies.









Similar content being viewed by others
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdel-Daim MM, Eissa IAM, Abdeen A, Abdel-Latif HMR, Ismail M, Dawood MAO, Hassan AM (2019) Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ Toxicol Pharmacol 69:44–50
Adebayo OA, Akinloye O, Adaramoye OA (2018): Cerium oxide nanoparticle elicits oxidative stress, endocrine imbalance and lowers sperm characteristics in testes of balb/c mice. Andrologia 50
Ahmed MM, Hussein MMA (2017) Neurotoxic effects of silver nanoparticles and the protective role of rutin. Biomed Pharmacother 90:731–739
Amann RP (2008) The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl 29:469–487
Barazani Y, Katz BF, Nagler HM, Stember DS (2014) Lifestyle, environment, and male reproductive health. Urol Clinics N Am 41:55–66
Bhattacharya T, Maishu SP, Akter R, Rahman MH, Akhtar MF, Saleem A, Bin-Jumah M, Kamel M, Abdel-Latif MA, Abdel-Daim MM (2021) A review on natural sources derived protein nanoparticles as anticancer agents. Curr Top Med Chem 21:1014–1026
Breznan D, Das DD, O’Brien JS, MacKinnon-Roy C, Nimesh S, Vuong NQ, Bernatchez S, DeSilva N, Hill M, Kumarathasan P, Vincent R (2017) Differential cytotoxic and inflammatory potency of amorphous silicon dioxide nanoparticles of similar size in multiple cell lines. Nanotoxicology 11:223–235
Cheng CY, Mruk DD (2012) The blood-testis barrier and its implications for male contraception. Pharmacol Rev 64:16–64
Croissant JG, Fatieiev Y, Khashab NM (2017) Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Advanced materials 29
El-Sayed A, Kamel M (2020a) Advanced applications of nanotechnology in veterinary medicine. Environ Sci Pollut Res Int 27:19073–19086
El-Sayed A, Kamel M (2020b) Advances in nanomedical applications: diagnostic, therapeutic, immunization, and vaccine production. Environ Sci Pollut Res Int 27:19200–19213
Gao G, Ze Y, Zhao X, Sang X, Zheng L, Ze X, Gui S, Sheng L, Sun Q, Hong J, Yu X, Wang L, Hong F, Zhang X (2013) Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J Hazard Mater 258–259:133–143
Garcia TX, Costa GM, Franca LR, Hofmann MC (2014) Sub-acute intravenous administration of silver nanoparticles in male mice alters Leydig cell function and testosterone levels. Reprod Toxicol 45:59–70
Hansen SF, Michelson ES, Kamper A, Borling P, Stuer-Lauridsen F, Baun A (2008) Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology 17:438–447
Huleihel M, Lunenfeld E (2004) Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J Androl 6:259–268
Hussein MMA, Gad E, Ahmed MM, Arisha AH, Mahdy HF, Swelum AA, Tukur HA, Saadeldin IM (2019) Amelioration of titanium dioxide nanoparticle reprotoxicity by the antioxidants morin and rutin. Environ Sci Pollut Res Int 26:29074–29084
Ige SF, Olaleye SB, Akhigbe RE, Akanbi TA, Oyekunle OA, Udoh UA (2012) Testicular toxicity and sperm quality following cadmium exposure in rats: ameliorative potentials of Allium Cepa. J Hum Reprod Sci 5:37–42
Kabir MT, Rahman MH, Akter R, Behl T, Kaushik D, Mittal V, Pandey P, Akhtar MF, Saleem A, Albadrani GM, Kamel M, Khalifa SAM, El-Seedi HR, Abdel-Daim MM (2021) Potential role of curcumin and its nanoformulations to treat various types of cancers. Biomolecules 11
Kandeil MA, Mohammed ET, Hashem KS, Aleya L, Abdel-Daim MM (2020) Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environ Sci Pollut Res Int 27:19169–19184
Kane L, Ismail N (2017) Puberty as a vulnerable period to the effects of immune challenges: focus on sex differences. Behav Brain Res 320:374–382
Khanna P, Ong C, Bay BH, Baeg GH (2015) Nanotoxicity: an interplay of oxidative stress, inflammation and cell death. Nanomaterials (basel) 5:1163–1180
Kong L, Tang M, Zhang T, Wang D, Hu K, Lu W, Wei C, Liang G, Pu Y (2014) Nickel nanoparticles exposure and reproductive toxicity in healthy adult rats. Int J Mol Sci 15:21253–21269
Korah N, Smith CE, D’Azzo A, Mui J, Hermo L (2003) Characterization of cell- and region-specific abnormalities in the epididymis of cathepsin A deficient mice. Mol Reprod Dev 66:358–373
Kusaczuk M, Kretowski R, Naumowicz M, Stypulkowska A, Cechowska-Pasko M (2018) Silica nanoparticle-induced oxidative stress and mitochondrial damage is followed by activation of intrinsic apoptosis pathway in glioblastoma cells. Int J Nanomed 13:2279–2294
Lafuente D, Garcia T, Blanco J, Sanchez DJ, Sirvent JJ, Domingo JL, Gomez M (2016) Effects of oral exposure to silver nanoparticles on the sperm of rats. Reprod Toxicol 60:133–139
Lee JA, Kim MK, Paek HJ, Kim YR, Kim MK, Lee JK, Jeong J, Choi SJ (2014) Tissue distribution and excretion kinetics of orally administered silica nanoparticles in rats. Int J Nanomed 9(Suppl 2):251–260
Leung CC, Yu IT, Chen W (2012) Silicosis Lancet 379:2008–2018
Li X, Yao Z, Yang D, Jiang X, Sun J, Tian L, Hu J, Wu B, Bai W (2020) Cyanidin-3-O-glucoside restores spermatogenic dysfunction in cadmium-exposed pubertal mice via histone ubiquitination and mitigating oxidative damage. J Hazard Mater 387:121706
Liu Y, Li H, Xiao K (2016) Distribution and biological effects of nanoparticles in the reproductive system. Curr Drug Metab 17:478–496
MacEwan DJ (2002) TNF ligands and receptors–a matter of life and death. Br J Pharmacol 135:855–875
Mathias FT, Romano RM, Kizys MM, Kasamatsu T, Giannocco G, Chiamolera MI, Dias-da-Silva MR, Romano MA (2015) Daily exposure to silver nanoparticles during prepubertal development decreases adult sperm and reproductive parameters. Nanotoxicology 9:64–70
Mital P, Hinton BT, Dufour JM (2011) The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod 84:851–858
Mohammed ET, Safwat GM (2020) Grape seed proanthocyanidin extract mitigates titanium dioxide nanoparticle (TiO2-NPs)-induced hepatotoxicity through TLR-4/NF-kappaB Signaling pathway. Biol Trace Elem Res 196:579–589
Mohammed ET, Hashem KS, Abdelazem AZ, Foda F (2020) Prospective protective effect of ellagic acid as a SIRT1 activator in iron oxide nanoparticle-induced renal damage in rats. Biol Trace Elem Res 198:177–188
Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, Donaldson K (2007) The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med 64:609–615
Morgan AMAE-HM, Noshy PA (2015) Reproductive toxicity investigation of titanium dioxide nanoparticles in male albino rats. J Pharm Pharm Sci 4(10):34–49
Morishita Y, Yoshioka Y, Satoh H, Nojiri N, Nagano K, Abe Y, Kamada H, Tsunoda S, Nabeshi H, Yoshikawa T, Tsutsumi Y (2012) Distribution and histologic effects of intravenously administered amorphous nanosilica particles in the testes of mice. Biochem Biophys Res Commun 420:297–301
Murugadoss S, Lison D, Godderis L, Van Den Brule S, Mast J, Brassinne F, Sebaihi N, Hoet PH (2017) Toxicology of silica nanoparticles: an update. Arch Toxicol 91:2967–3010
Nemmar A, Yuvaraju P, Beegam S, Yasin J, Kazzam EE, Ali BH (2016) Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int J Nanomed 11:919–928
Parveen A, Rizvi SH, Sushma MF, Ahmad I, Singh PP, Mahdi AA (2017) Intranasal exposure to silica nanoparticles induces alterations in pro-inflammatory environment of rat brain. Toxicol Ind Health 33:119–132
Qian Q, Cao X, Wang B, Qu Y, Qian Q, Sun Z, Feng F (2019) TNF-alpha-TNFR signal pathway inhibits autophagy and promotes apoptosis of alveolar macrophages in coal worker’s pneumoconiosis. J Cell Physiol 234:5953–5963
Saleh RA, Agarwal A (2002) Oxidative stress and male infertility: from research bench to clinical practice. J Androl 23:737–752
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Shang X, Shen C, Liu J, Tang L, Zhang H, Wang Y, Wu W, Chi J, Zhuang H, Fei J, Wang Z (2018) Serine protease PRSS55 is crucial for male mouse fertility via affecting sperm migration and sperm-egg binding. Cell Mol Life Sci : CMLS 75:4371–4384
Skovmand A, Jensen ACO, Maurice C, Marchetti F, Lauvas AJ, Koponen IK, Jensen KA, Goericke-Pesch S, Vogel U, Hougaard KS (2019) Effects of maternal inhalation of carbon black nanoparticles on reproductive and fertility parameters in a four-generation study of male mice. Part Fibre Toxicol 16:13
Takahashi O, Oishi S (2001) Testicular toxicity of dietary 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in F344 rats. Arch Toxicol 75:42–51
Tremellen K (2008) Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update 14:243–258
Wallach D, Kang TB, Kovalenko A (2014) Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol 14:51–59
Wang R, Song B, Wu J, Zhang Y, Chen A, Shao L (2018) Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomedicine 13:8487–8506
Winkler HC, Notter T, Meyer U, Naegeli H (2018) Critical review of the safety assessment of titanium dioxide additives in food. J Nanobiotechnology 16:51
Xu Y, Wang N, Yu Y, Li Y, Li YB, Yu YB, Zhou XQ, Sun ZW (2014) Exposure to silica nanoparticles causes reversible damage of the spermatogenic process in mice. PloS one 9:e101572
Zahin N, Anwar R, Tewari D, Kabir MT, Sajid A, Mathew B, Uddin MS, Aleya L, Abdel-Daim MM (2020) Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res Int 27:19151–19168
Zhang R, Zhang L, Jiang D, Zheng K, Cui Y, Li M, Wu B, Cheng S (2014) Mouse organ coefficient and abnormal sperm rate analysis with exposure to tap water and source water in Nanjing reach of Yangtze River. Ecotoxicology 23:641–646
Zhang J, Ren L, Zou Y, Zhang L, Wei J, Li Y, Wang J, Sun Z, Zhou X (2016) Silica nanoparticles induce start inhibition of meiosis and cell cycle arrest via down-regulating meiotic relevant factors. Toxicol Res 5:1453–1464
Zhang X, Luan J, Chen W, Fan J, Nan Y, Wang Y, Liang Y, Meng G, Ju D (2018) Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis. Nanoscale 10:9141–9152
Zhang L, Wei J, Duan J, Guo C, Zhang J, Ren L, Liu J, Li Y, Sun Z, Zhou X (2020) Silica nanoparticles exacerbates reproductive toxicity development in high-fat diet-treated Wistar rats. J Hazard Mater 384:121361
Zhao H, Gu W, Ye L, Yang H (2014) Biodistribution of PAMAM dendrimer conjugated magnetic nanoparticles in mice. Journal of materials science. Mater Med 25:769–776
Zhou Q, Yue Z, Li Q, Zhou R, Liu L (2019) Exposure to PbSe nanoparticles and male reproductive damage in a rat model. Environ Sci Technol 53:13408–13416
Acknowledgements
We would like to thank Fang Yang and Hong Xu for the technical assistance in western blot technique, Heliang Liu and Xiaohui Hao for the generous contribution of antibodies, and many of our colleagues for providing valuable advices on the experiments and manuscript.
Funding
This work was supported by the grants from the Natural Science Foundation of Hebei Province (no. C2021209004), the grants from the Tangshan Science and Technology Bureau (no. 20130212b), the grants from the Science and Technology Project of Hebei Education Department (no. JQN2020002), the grants from the Department of Health of Hebei Province (no. 20210049), and the grants from the Undergraduate Innovation and Entrepreneurship Project of North China University of Science and Technology (no. X2020085).
Author information
Authors and Affiliations
Contributions
XS and FS conceived and designed the study; XS, FS, XW, ZC, and PZ performed experiments and interpreted data; XS and ZG drafted the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were reviewed and approved by the Laboratory Animal Ethics Committee of North China University of Science and Technology.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, F., Wang, X., Zhang, P. et al. Reproductive toxicity investigation of silica nanoparticles in male pubertal mice. Environ Sci Pollut Res 29, 36640–36654 (2022). https://doi.org/10.1007/s11356-021-18215-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18215-6