Abstract
Anthropogenic factors are contaminating crystalline aquifers more rapidly than natural sources and affecting human health in many states in India. Since a large population depends on untreated groundwater, identifying the sources of this contamination and assessing the related human health risk are essential to ensure a good-quality water supply. Nitrate is one of the most widespread means of groundwater contamination in many parts of India. Coimbatore and Tirupur districts are the most rapidly growing industrial urban areas in southern India. This paper deals with nitrate contamination and possible health risks for children and adults in the Coimbatore and Tirupur districts based on 93 groundwater samples. To achieve this goal, classical hydrochemical and deterministic hazard identification methods coupled with spatial mapping technologies were applied. A wide variation in nitrate concentration, between 1 and 415 mg/L, was observed, with 37% of the samples exceeding the WHO permissible limit of 50 mg/L. The distinct concentrations of nitrate and other ions observed spatially can be attributed to the diverse geochemical and land use settings in the study area. The bivariate plots of NO3 with other ions suggested that the principal origin of nitrate in this study is related to the excess application of fertilizers and sewages. The spatial variation of NO3, in comparison with the land use map, confirmed these results. The values of hazard quotient (HQ) via ingestion exceeded the critical value, one in 40% in males, 42% in females, and 45% in children. However, HQ values via oral pathways are within one and pose no exposure risk. Thus, the hazard index corresponds to HQingestion only. The health risk was in the increasing order of male>female>children, and shows that body weight is the most critical factor that is influencing the health impact to children as compared to adults. The spatial variation of hazard index values showed that groundwater quality is highly polluted with NO3 in the north and northeastern parts of the study area, mainly due to intensive agricultural practices, and poses critical health concerns. Considering the increasing population and higher dependencies on groundwater, immediate and sufficient measures are proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater is a vital source of water which supports human health, agriculture, and industrial development and plays a critical role in the sustainability and functioning of ecosystems due to a lack of clean surface water (Ali and Ali 2018; Steube et al. 2009). The industrial revolution and the rise in anthropogenic activities over the past decades have increased the need for groundwater more than ever before (Kaviarasan et al. 2016). The uncontrolled growth in population provokes a rapid increase in socioeconomic activities, which may emit contaminants into the environment, thus posing a threat to groundwater quality. Fertilizers, effluent runoff from industries, chemical dumping, and domestic sewage are considered to be the primary sources of groundwater pollutants and waterborne diseases (Nalbantcilar and Pinarkara 2016). Approximately 25,000 people around the globe die every day due to lack of water or consumption of polluted water, and nearly one-third of the urban population in developing countries lacks safe drinking water (Li and Ling 2006). Of all the groundwater pollutants, NO−3 (nitrate) has emerged as one of the most dangerous and widespread contaminants of groundwater in arid and semi-arid regions (Adimalla 2019; Chen et al. 2016; Chica-Olmo et al. 2016; Li et al. 2019).
Nitrate is a form of nitrogen and originates naturally in the environment from atmospheric nitrogen, final ion by plants, and lightning storms. It can also appear as the result of anthropogenic activities like fertilizers, septic tanks, sewage, and improper use of animal manures for agriculture. NO3 is innocuous to human beings, and its formation is a fundamental part of the nitrogen cycle (Shukla and Saxena 2018). Nitrate, which is ingested through drinking water, however, is reduced from nitrate to nitrite in the gut by bacteria. This causes methemoglobinemia, or more generally known as a blue baby syndrome in new-born babies, a life-threatening condition that decreases the ability of blood to carry oxygen through the body. Nitrite, converted from nitrate, can also react with organic compounds to form amines and amides through intrastation in the stomach, and the interaction of nitrous acid with secondary and tertiary amides, amines, and nitrogen-containing compounds forms N-nitroso compounds, widely considered to cause gastric cancer. In addition, there are many other diseases such as thyroid dysfunctions, breathing problems, nuisance and tiredness, and multiple sclerosis that can result from high nitrate intake (Ahada and Suthar 2018; Gatseva and Argirova 2008; Vanhatalo et al. 2018; WHO 2011).
Groundwater NO−3 contamination is highly discussed around the globe and has become a predominant threat because of the uncontrolled use of pesticides (Shukla and Saxena 2018; Spalding and Exner 1993). In India, nitrate contamination is an emerging issue regarding groundwater safety and human health. It is reported that one-third of the states in India have excess nitrate in their drinking water, and Tamil Nadu is one of them (NEERI 1991; Taneja et al. 2017). Nitrate fertilizer usage in India has tremendously increased over a period of 55 years from 1951 to 2006 (Adimalla 2019). Because of a low level of retentiveness of NO−3 by the soil and a high level of water solubility, the excess amount of NO−3, unutilized by the plant, gets easily leached to the sub-soil layer. This leached NO3 is finally reaching groundwater table and traveling in the same velocity of groundwater (Ahada and Suthar 2018). Nitrate concentration is very high in arid and semi-arid areas, though much lower in deep groundwater as compared to shallow groundwater (He et al. 2020).
Many studies have been done on the risk to human health associated with nitrate-contaminated groundwater in India. Adimalla (2019) studied the exposure risk and health impacts of nitrate in Nirmala province, South India, and reported that the health of the women and children are profoundly affected. Ahada and Suthar (2018) carried out a study in the southern district of Punjab to assess NO3 contamination and showed that 92% of the area has a higher level of NO3 than the safe limit. Another study in the Shanmunganadhi river basin also reported that children are more vulnerable than adults for health risks from elevated levels of nitrate (Karunanidhi et al. 2019).
According to the WHO, the maximum allowable limit of nitrate in drinking water is 50 mg/L (WHO 2017). A higher concentration of nitrate than this level has already been reported in some parts of the area covered in this study (Sajil Kumar et al. 2014). However, no study covering the entire Coimbatore and Tirupur district has been published to date. The need for this study is essential because there is a growing need for greater agricultural activities to feed an increasing population. The population growth is promoting urbanization and exponentially increasing settlements in the study area. All these factors affect nitrate levels in groundwater and deteriorate the groundwater quality. In this background, a detailed study was conducted to understand the track of nitrate and to determine the human health risk of nitrate exposure for children and adults (men and women). Furthermore, to quantify the quality of the groundwater, a drinking water quality index (DWQI) was developed. Thus, this data can be used as an assessment guideline for the decision-makers in groundwater quality protection and management.
Study area settings
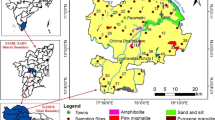
The location map of the study area and the sampling wells are shown in Fig. 1. Coimbatore district is bordered by the Western Ghats in the west and Tirupur district in the eastern boundary. According to the CGWB (2008) report, the climate of this region is sub-tropical, with a range of temperatures from 14 to 40 °C. The primary rainy season is the SW and NE monsoon seasons which together contribute around 650 mm of rainfall per year. Bhavani, Noyyil, and Amaravathy are the main rivers, but are usually dry in the summer season. The topography is mostly undulating, and the slope of the terrain is mainly to the east. The lithology of the Coimbatore district is comprised of metamorphic rocks, charnockites, granites, hornblende–biotite gneiss, sillimanite gneiss with basic and ultra-basic intrusive, crystalline limestone, syenite, pegmatite, and quartz veins. Sedimentary formations such as alluvium and kankar deposits are found in the Noyyal and Bhavani river basins. Of the rock types, hornblende–biotite gneiss is the most abundant. The geomorphology of the district includes structural and residual hills, linear ridges, Bazada zones, buried piedmonts, active pediments, shallow piedmonts, erosional plains, and valley fills (CGWB 2008). Red calcareous soil, black soil, red non-calcareous soil, alluvial and colluvial soil, brown soil, and forest soil are the common soil types. Most of the aquifers are crystalline rocks from the Archean age and with water stored in the fissures, fractures, joints, and weathered portions. Along the Noyyal river course, the sedimentary formations also act as water-bearing formations. Weathered hard rocks are up to 30 m thick and are the prominent water storage medium in the study area.
Tirupur is the nearby district of Coimbatore district which is added at a later stage to the state of Tamil Nadu. The climate is also tropical in nature with a temperature of 20 to 40 °C. The district receives little rainfall as it is situated along the leeward side of the Western Ghats (Arumugam and Elangovan 2009). The NE monsoon is the most important source of groundwater recharge as the SW rains are negligible. Lithology follows the same pattern as in Coimbatore. Dug wells and bore wells are the essential methods of water extraction. In the past few decades, the subsidized power supply for the farming villages has affected the groundwater table through the overexploitation of groundwater resources.
Materials and methods
Groundwater sampling and analytical techniques
A total of 93 groundwater samples were collected in July of 2016 from the Coimbatore and Tirupur districts in Tamil Nadu and were analyzed at the water quality laboratory of Institute of Water Studies (IWS), Chennai. These wells were selected after a reconnaissance survey which considered the importance of representing the exact hydrogeological condition of the aquifers. Water was pumped out before sampling to stabilize the physicochemical parameters. Samples were collected in pre-cleaned polyethylene bottles, sealed, and brought to the laboratory for analysis. Samples were kept at 4 °C until the analysis was completed. Standard analytical procedures, as suggested by APHA (1995) for the analysis of groundwater samples, were followed throughout. Only highly pure (AnalR Grade) chemicals and double-distilled water were used for preparing solutions for analysis. Electrical conductivity (EC) and pH were analyzed using portable digital meters in the field. Calcium (Ca2+) and magnesium (Mg2+) were determined using EDTA titration. Chloride (Cl−) was determined by standard AgNO3 titration. Carbonate (CO32−) and bicarbonate (HCO3−) were determined by titration with HCl. Sodium (Na+) and potassium (K+) were measured by flame photometry. Sulfate (SO42−) was determined by using a UV–visible spectrophotometer. Nitrate was measured by ion chromatography. The values were observed to be within the standard limit of ± 5%.
Drinking water quality index
Based on the physicochemical analysis of pH, total dissolved solids (TDS), NO3, Ca, Mg, Na, K, Cl, SO4, CO3, HCO3, F, and TH, the DWQI was calculated. These parameters were assigned weights (wi) between 1 and 5, according to their importance and health effects (Sajil Kumar et al. 2019). The highest weight of five has been given to F and NO3¯, as they are highly toxic even with very low concentration. A weight of four has been assigned to pH, Cl, Na, and Ca, and the other parameters were assigned a medium weight of three or less. Based on the assigned weights, the relative weights for each parameter are calculated using Eq. 1, and the values are shown in Table 1.
In this equation,
- RWi:
-
is the relative weight
- Wi:
-
is the rate of each parameter
- n:
-
is the number of parameters
In the next step, the quality rating scale (qi) has been calculated using Eq. 2
In this equation,
- qi:
-
is the quality rating
- Ci:
-
is the concentration of ions in mg/L
- Si:
-
is the drinking water quality standard (WHO 2017)
Then, the sub-index (SI) was calculated using Eq. 3, allowing the DWQI to be calculated with Eq. 4.
Human health risk assessment
The toxic elements affect human health, mainly by inhalation, ingestion, and dermal pathways. A high concentration of nitrate in water can cause serious health issues. In this study, the health impact of nitrate on males, females, and children has been investigated. The standard procedures and reference values suggested by the United State Environmental Protection Agency (USEPA 2001) are used in the health risk assessment. Exposure risks by ingestion and dermal pathways were calculated using Eq. 5 and Eq. 6:
Here, CDDIN and CDDDE represent the chronic daily dose by ingestion and dermal effects, respectively (μg/kg day). Cwater is the concentration of NO3 in groundwater (mg/L). IR is the ingestion rate of water in L/day (adults = 2.5 L/day; children = 0.78 L/day). SA is the exposed skin area in cm2 (adults = 16,600 cm2; children = 12,000 cm2). KP is the dermal permeability coefficient for water (0.001, no unit). EF is the water exposure frequency (365 days). ED is the exposure duration in years (males = 64, females = 67, and children = 12,). ET is the water exposure time in hours/day (0.4 h/day for adults and children). BW is the body weight in kg (men = 65, women = 55, and children = 15). AT is the average residence time measured in days/year. CF is the conversion factor (0.001 for adults and children).
The hazard quotient (HQ) of NO3 exposure via ingestion and dermal pathways can be calculated using Eq. 7 and Eq. 8 below:
HQIN is the ingestion-based hazard quotient. HQDE is the dermal-based hazard quotient. RfD is the reference dose of NO3, i.e., 1.6 mg/kg/day (USEPA 2014).
Hazard index (HI) is the overall risk of exposure via both digestion and dermal pathways.
If the HI values are higher than one, it may cause non-carcinogenic health hazards.
Spatial mapping using inverse distance weighting
The distribution of ions in different locations can be easily visualized and interpreted by plotting them on spatial variation maps. The spatial analyst tool in ArcGIS has several interpolation techniques. One of the easiest and useful methods is inverse distance weighting (IDW), used in this study. The basic assumption is that when several known points are distributed through space, the points near them are mostly similar than those falling away. The main advantage is that the method is logical and efficient. Since our sample points are mostly evenly distributed, this study has adopted the IDW method. The weights for unknown points may be determined using the following equation,
where λi is the weight of point, Di is the distance between point “i” and the unknown point, and α is the power ten of weight.
Results and discussion
Groundwater quality
The statistical summary of the major physio-chemical parameters and their permissible limit for drinking purpose is shown in Table 2. One of the significant operational water quality parameters is pH, which requires an optimum range of 6.5 to 8.5 (WHO 2017). In the samples from this study, pH varied between 7 and 9, with a mean value of 8. This indicates that the groundwater of the study region is slightly alkaline. The pH value is controlled by carbonates and bicarbonates in the aquifer (Saha and Paul 2019). EC indicates the total amount of dissolved salts in the water; in physical terms, it is the capacity of water to convey electric current. In the present study, EC ranged from 180 to 7290 μS/cm, with an average value of 1811 μS/cm. TDS measure the total concentration of dissolved substances in the water. TDS is a combination of inorganic salts and a small amount of organic matter (Li et al. 2008; Sajil Kumar 2014). TDS values range from a minimum value of 100 to a maximum value of 4439 mg/L. The average value of TDS in the present study is 1103 mg/L. According to the classification of TDS suggested by Todd (1980), samples 91, 62, and 4 fall under the category of very freshwater (0–250 mg/L); 60% of samples fall under the category of freshwater (250–1000 mg/L); and 37% of sample fall under the category of brackish (1000–10,000 mg/L), with none of the samples in the category of saline (10,000 to 100,000 mg/L).
In major anions, the order of abundance was found to be Cl˃HCO3−˃SO4−˃NO3−˃F−. Chloride in the groundwater may generally come from anthropogenic sources such as runoff containing road de-icing salts, inorganic fertilizer usage, effluents from septic tanks and industrial activities, animal feeds, and seawater intrusion in coastal areas (Han et al. 2014). The value of chloride in the present study was observed to be between 14 and 2092 mg/L with a mean value of 275 mg/L, which is above the acceptable limit of 250 mg/L (WHO 2017). A high concentration of chloride gives drinking water a salty taste. Bicarbonate concentration varied between 69 and 604 mg/L, with an average value of 277 mg/L. The concentration of bicarbonate remained mostly within the limit of 500 mg/L. The sulfate concentration varied between 2 and 816 mg/L, with an average of 116 mg/L. Samples 1, 11, 35, 40, 41, 44, 47, 53, 75, and 81 were found to exceed the allowable limit of 250 mg/L (WHO 2011). The range of fluoride in groundwater was between 0 and 2.0 mg/L, with an average of 1 mg/L. Together, 19% of the total samples exceed the permissible limit of 1.5 mg/L (WHO 2011).
The dominance of the major cations was in the order of Na+˃Ca+˃Mg2+ ˃K. Na is the dominant ion among the cations, and its concentration ranged between 7 and 690 mg/L, with a mean value of 146 mg/L. The permissible limit of Na+ in drinking water is 200 mg/L. In total, 24% of the samples showed that the level of Na+ exceeded this permissible limit. Sample 75 showed the highest value, measuring 690 mg/L. Ca+ and Mg2+ directly contribute to water hardness and are the most abundant elements in groundwater. The Ca and Mg concentrations in the water samples from the present study varied from 10 to 700 mg/L, avg. 87 mg/L, and 6 to 292 mg/L, avg. 75 mg/L, respectively. The Ca2+concentration of all samples, except samples 11, 13, and 81, was within the permissible limit of 300 mg/L. The permissible limit for Mg2+ of 150 mg/L was exceeded by 15% of the total samples. K+ varied between 4 and 360 mg/L, with an average of 43 mg/L, remarkably higher than the permissible limit of 12 mg/L.
Total hardness is defined as the sum of calcium and magnesium ions in water. In the present study, the lowest value of 60 mg/L was found in sample 61 and the highest value of 2000 mg/L was found in sample 11. The average value of the total hardness was 527 mg/L, which is higher than the desirable limit of 300 mg/L. According to Sawyer (1967), 30% of the samples fall under the category of hard (150–300 mg/L) and 70% under the category of very hard (˃ 300 mg/L), while none was found to fall under the category of soft (0–75 mg/L) or moderately hard (75–150 mg/L).
Drinking water quality index
DWQI or drinking water quality index is a mathematical operative tool which presents a single number for the overall quality of drinking water (Mohebbi et al. 2013). This can help in the decision-making process of administrative representatives for better planning and management of groundwater resources (Sajil Kumar et al. 2019). According to the DQWI method, the physicochemical analysis data of the present study was classified into excellent water (˂ 50), good water (50–100), poor water (100–200), very poor water (200–300), and water unsuitable for drinking when the DWQI exceeds the value of 300 (Mohebbi et al. 2013). A detailed classification of the DWQI for this study is given in Table 3.
In the present study, the computed DWQI value ranges between 26 and 361. The average value of the DWQI is 114, which is classified as poor water quality for drinking purposes. In total, 23% of the samples have DWQI values below 50, indicating excellent quality, and 36% of the samples ranged between 51 and 99, categorized as good water. On the other hand, 26% of the samples showed very poor quality for drinking purposes. Sample 1 at Timmanaickanpalayam village gave the highest value of 361, followed by sample 11 at Pudupalayam village with the value 319. In sample 1, the DWQI value was high due to the high level of TDS (2173 mg/L), and other ions such as NO3 (366 mg/L), Cl (425 mg/L), SO4 (260 mg/L), and K (360 mg/L). The presence of a high concentration of NO3 and K shows that the pollutants originate from agricultural fertilizers.
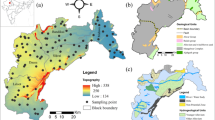
The spatial variation map of the DWQI showed that the samples in the southern, eastern, and northeastern regions have comparably better-quality water than the northern and northwestern regions (Fig. 2). Some samples in the central part and majority of the samples in the northwestern region have very high DWQI values, categorized as poor or very poor. The samples in these regions are mostly affected by anthropogenic inputs such as industrial or agricultural activities. The land use pattern of the study area agrees with this inference.
Groundwater types
A Durov diagram can reflect general geochemical processes in groundwater (Durov, 1948) (Fig. 3). It is also useful in identifying the hydrochemical types of different water samples (Li et al. 2014). In this study, most of the samples were in fields 2, 3, 5, 6, and 8. Exceptionally few samples were plotted in field 7. Field 1 shows the natural groundwater chemistry dominated by Ca and HCO3. As it goes from field one to three, significant ion exchange between the cations is expected. Field 2 generally represents the dominance of Ca and Mg along with HCO3, while in field 3, Na and K dominate the cations and HCO3 the anions. In this study, nine samples fall in field 2, reflecting the dominance of Ca and Mg, while three samples fall in field 3, showing the control of Na and K ions. Many samples were also plotted in field 5, which indicates simple mixing or dissolution processes; in this group, there is no dominance of any anions or cations (Chen et al. 2019). Field 7 shows the dominance of Ca and Cl, generally due to the reverse ion exchange process of Ca from groundwater with Na in the aquifer matrix, while field 8 has a dominance of Ca and Mg along with Cl in several samples, which is indicating the mixing of saline water with the freshwater.
Nitrate concentration and possible origins in groundwater
The levels of nitrate in groundwater varied between 1 and 415 mg/L, with the average value being 77 mg/L. The permissible limit of nitrate in drinking water, as per WHO guidelines, is 50 mg/L (WHO 2017). Of the 92 samples, 34 exceeded this permissible level for drinking purposes. However, the standard Indian value is lower, at 45 mg/L, making a total 35 samples exceeding this limit. Drinking water standards may vary from place to place depending on water intake related to climatic conditions.
The origin of nitrate is rarely reported from the geological formations. However, the possible origin of nitrate via microbial nitrification can be proposed as the only source of nitrate from natural processes. In this process, ammonia is initially converted to nitrite and then to nitrate with bacterial activity, as shown in Eq. 10 and Eq. 11.
An abnormal level of nitrate concentration (> 10 mg/L) must originate from anthropogenic inputs (Panno et al. 2006). The non-point sources, such as agricultural practices, and localized factors, such as sewage leakages, generally control nitrate concentration in surface- or shallow- level groundwater (Wang et al. 2017). It has been observed that higher levels of contamination are mostly associated with shallow groundwater and are the result of cultivation models, manures, wastewater irrigation, redox conditions, precipitation surpluses, waste disposal networks, animal wastes, industry, river–aquifer interactions, denitrification, and related biogeochemical processes in groundwater (Jia et al. 2020; Taufiq et al. 2019; Zendehbad et al. 2019). In the study area, farming activities and farm animals could be the biggest sources of nitrate in groundwater. A large quantity of fertilizer and manures are used in the farming period, usually from February to August, and are leaked into the groundwater with the precipitation surplus. Another interesting source of nitrate was reported by Wakida and Lerner (2005), urbanization and the construction of new buildings which are likely to disturb the soil and soil aeration and develop an atmospheric contract with the nitrogen-fixing microorganisms. The net result if this may increase nitrate mineralization in soil and then increase levels in groundwater.
Ionic correlation plots were drawn to identify the sources of NO3 in the study area. In this plot, there are many samples that have positively varied for NO3 with respect to K, SO4, and Cl (Fig. 4). The samples with a positive correlation for NO3 with K and SO4, indicating the origin of these ions, derived from a non-point source of pollution, including fertilizers (Papazotos et al. 2019). While several samples do not show any positive relation, nitrate is generally controlled by geogenic factors, typically rock–water interaction and weathering of silicate or sulfate minerals. The low concentrations of K in some places can be attributed to the adsorption of K ions by clay minerals (Sajil Kumar et al. 2014).
Similarly, in the inland regions, the presence of Cl ions in groundwater is an indicator of anthropogenic pollution. There are several samples plotted away from the 1:1 line of NO3 vs. Cl, indicating that the origin is not the same for these ions in those samples. The primary source of Cl in the study area is industrial effluents rich in common salt (NaCl), and it is reflected from the substantially high positive concentration for Na and Cl in some of the samples. Furthermore, a very high positive correlation between Na and Cl in Fig. 5 confirms the origin of salinity as highly saline effluent wastewater. However, other sources of Na, like rock weathering, contribute to the total sodium concentration in water, which is evident from some of the samples in which there is no correlation for Na and Cl. These samples represent the natural origin of Na and the possibility of cation exchange with Ca, as observed in the Durov plot. On the other hand, there is a positive correlation of Cl in some of the samples with NO3, indicating their common origin from animal and human wastes (Wang et al. 2017). In Fig. 6, the relationship between Mg and Cl+NO3 is plotted to understand the origin of NO3. An excellent positive correlation between these ions suggests the contribution of agrochemical products (Papazotos et al. 2019). In this plot, it can be observed that the Mg concentrations in some of the samples are higher than the Cl+NO3 concentrations, reflecting other sources such as rock weathering.
Spatial variation of nitrate and land use influences
The primary land use patterns are agricultural and industrial with human settlements (Fig. 7). Tirupur is especially famous for textile production, and the exports from this region contribute a large portion of the country’s economy. On the other hand, these industries are polluting the environment, and many regions have been contaminated to an irreversible extent. Variation in nitrate concentration in the study area is shown in Fig. 8, where it can be observed that the concentration is within the permissible limit of 50 mg/L in the majority of the Coimbatore district, while, in contrast, large areas in the Tirupur district have a very high concentration of NO3 in groundwater. The spatial dissemination of NO3 is largely related to the fertilizer application rate and the efficiency of plants, cropping patterns and cycles, irrigation models, soil structures and textures, and finally, changes in regional climate (Jacks and Sharma 1983; Suthar et al. 2009). The spatial variation maps show that the eastern and northeastern parts of the study area have elevated NO3 concentrations.
It has been observed that the hilly regions (recharge areas) have low NO3 values; on the other hand, along the flow paths of the Noyyal river, the values are increasing. Nitrate levels in groundwater are mostly controlled by the land use patterns of the region (Lockhart et al. 2013). As shown in Fig. 7, the land use map of the study area shows that large portions of the study area are used primarily for agricultural activities. Forest land and human settlements are the next most prominent land uses. In agricultural areas, it can be observed that the application of fertilizer is higher than the required level. In this study area, the surface irrigation is generally practiced, which results in uneven distribution of water and sometimes results in irrigation return flows. Growing crops like paddy, maize, vegetables, and grain needs seasonal tillage, which alters the soil structure and its ability to activate microbial fixation of nitrate, especially in the bean-cultivating regions. The human settlements in the eastern part of the study area are the result of the fertile soil for agriculture (SPA 2008). In addition, the exponential growth of settlements generates a substantial amount of human and animal waste, also causing nitrate contamination in the groundwater of the study area. In several locations, sanitation facilities are inadequate or unavailable. It is causing the leaching of nitrate into soil, which is partially consumed by plants and finally reaching the groundwater.
Human health risk assessment of nitrate
Health risk assessment is the quantification of harmful health effects in humans due to continuous excess exposure to toxic chemicals in the environment (Şener et al. 2017). Table 4 shows a summary of the statistics for the health risk assessment of nitrate for males, females, and children. The chronic daily intake (CDI) values of nitrate for males varied between 0.04 and 15.96 mg/kg/day, with an average of 2.96 mg/kg/day; CDI for females varied between 0.05 and 18.87 mg/kg/day, with an average of 3.5 mg/kg/day, and for children, it was between 0.05 and 21.58 mg/kg/day with an average of 4.00 mg/kg/day. CDI values were further used in determining the hazard quotient via ingestion.
The dermally absorbed dose (DAD) was calculated as a step of determining the HQDermal. DAD values of nitrate for males varied between 0.0 and 0.04 mg/kg/day, with an average of 0.01 mg/kg/day. DAD for females varied between 0.0 and 0.05 mg/kg/day, with an average of 0.01 mg/kg/day. For children, DAD ranged between 0.00 and 0.13 mg/kg/day with an average of 0.02 mg/kg/day.
In this study, HQ through the oral pathway for men varied between 0.024 and 9.9. The average value is 1.85, which is higher than the recommended value of 1; thus, a non-carcinogenic risk is visible for men in the study area. Sample 36 from the Munduelanpatty village showed the highest value at 9.9, followed by sample 1 in the Timmanaickanpalayam village which was measured at 8.79. From a total of 37 samples, 40% exceeded the permissible value. Slightly higher values, between 0.03 and 11.79, were observed in HQOral for females, with an average value of 2.19. Thirty-nine samples (42%) had an HQOral higher than 1 for females. Among the three categories studied, children have the highest values of HQOral, varying between 0.03 and 13.57, with an average of 2.52. In total, 44 samples (45%) exceeded the limit and pose severe health impacts for children. In this study, HQDermal was less than 1 in all the samples, and thus does not considerably impact human health.
As shown in Table 3, the cumulative HI of males ranges between 0.024 and 10.00, with an average of 1.90. In the case of females and children, the range is from 0.028 to 11.825 and 0.032 to 13.57 respectively, with a mean value of 2.24 for females and 2.57 for children. In the evaluation, the average cumulative hazard index for males, females, and children is higher than the recommended value of 1, thus revealing the high non-carcinogenic risk in the study area due to nitrate concentration. The value of HI for children is higher than for females or males and is in the order HIchild˃HIfemale˃HImale (Fig. 9). Therefore, it is clear that children are more vulnerable to non-carcinogenic risks due to nitrate toxicity. The basic reason for this is that their water consumption is more per unit of their body weight than adults (Chen et al. 2016; Li et al. 2019). The HI for children was found to be the highest in sample 36 (Munduvelampaty village), followed by sample 1 (Timmanckanpalayam village). Villages Tundarkarampalayam, Sevur, Kanjeerapalayam, Vellakoil, MuthunaickkenValasu, Periakumarapalayam, Uthgiyur, Kambliyampatti, AvinashipalayamSouth, Pongalur, Veerapadi, K.Ayyampalayam, and Vadavalli also showed very high values of HI for children.
The spatial variation of HI values was plotted for males, females, and children in Fig. 10a, b, and c. It can be observed from the plot that the groundwater quality is heavily polluted in the north and northeastern parts of the study area. As explained in the earlier section, this is mainly due to intensive agricultural practices. The spatial maps show that the percentage of critical regions was predicted to be higher than that observed in the statistical summary. From males to children, the values of HI were found to increase from the central part of the study area to the northeastern part, though the health risk can be expected to spread to the southern part of the region as the land use pattern of the study area changes due to government promotion of more intensive agricultural practices. This will mean an accelerated use of fertilizers and manures, which will then increase the NO3 concentration in the groundwater. In this situation, there is an urgent call for tackling the problem of ensuring a supply of safe drinking water for all the villages, a need which requires proper monitoring of water quality in contaminated sites.
Conclusions
Groundwater quality as related to nitrate contamination has been studied in the Coimbatore and Tirupur districts using classical methods such as bivariate plots and Durov diagrams. Based on the hydrochemistry of the groundwater samples, the results of this study support the existence of natural and anthropogenic influences on water quality. Nitrate levels in the study area varied between 1 and 415 mg/L, with an average value of 77 mg/L. In total, 37% of the samples exceeded the WHO drinking water limit of 50 mg/L. It is observed that the origin of nitrate can be primarily attributed to the overuse of agricultural fertilizers and to leaks in urban sewage and septic tanks resulting from exponential population growth. Risk from exposure to nitrate (HQDermal < 1 in all the samples) has not been found in the study area; however, there is a possible health risk due to ingestion. The HQ via ingestion exceeded one in 40% of males, 42% of females, and 45% of children. The HI was the same as the HQoral, as there are no health issues related to the dermal pathway. The order of human health risk is children˃females˃males; even with the same concentration of nitrate in drinking water, children are more prone to illness because body weight has been found to be the determining factor causing variation in health risk in these three categories. According to the spatial variation of HI values, the highest health risk exists in the north and northeastern parts of the study area and can be attributed to intensive agricultural practices.
Data availability
The datasets used and/or analyzed during the current study is available from the corresponding author on reasonable request.
Change history
20 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11356-021-17079-0
References
Adimalla N (2019) Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Hum Ecol Risk Assess Int J 1–25. https://doi.org/10.1080/10807039.2018.1508329
Ahada CPS, Suthar S (2018) Groundwater nitrate contamination and associated human health risk assessment in southern districts of Punjab, India. Environ Sci Pollut Res 25(25):25336–25347. https://doi.org/10.1007/s11356-018-2581-2
Ali SA, Ali U (2018) Hydrochemical characteristics and spatial analysis of groundwater quality in parts of Bundelkhand Massif, India. Appl Water Sci 8(1). https://doi.org/10.1007/s13201-018-0678-x
APHA (1995) Standard methods for estimation of water and waste water. American Public Health Association, Washington
Arumugam K, Elangovan K (2009) Hydrochemical characteristics and groundwater quality assessment in Tirupur Region, Coimbatore District, Tamil Nadu, India. Environ Geol 58(7):1509–1520. https://doi.org/10.1007/s00254-008-1652-y
CGWB (2008) District groundwater brochure Coimbatore district, Tamil Nadu. Technical report series
Chen J, Wu H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, Northwest China. Expo Health 8. https://doi.org/10.1007/s12403-016-0208-8
Chen J, Huang Q, Lin Y, Fang Y, Qian H, Liu R, Ma H (2019) Hydrogeochemical characteristics and quality assessment of groundwater in an irrigated region, Northwest China. Water 11(1):96. https://doi.org/10.3390/w11010096
Chica-Olmo M, Peluso F, Luque-Espinar JA, Rodriguez-Galiano V, Pardo-Igúzquiza E, Chica-Rivas L (2016) A methodology for assessing public health risk associated with groundwater nitrate contamination: a case study in an agricultural setting (southern Spain). https://doi.org/10.1007/s10653-016-9880-7
Gatseva PD, Argirova MD (2008) High-nitrate levels in drinking water may be a risk factor for thyroid dysfunction in children and pregnant women living in rural Bulgarian areas. Int J Hyg Environ Health 211(5–6):555–559. https://doi.org/10.1016/j.ijheh.2007.10.002
Han DM, Song XF, Currell MJ, Yang JL, Xiao GQ (2014) Chemical and isotopic constraints on evolution of groundwater salinization in the coastal plain aquifer of Laizhou Bay, China. J Hydrol 508:12–27. https://doi.org/10.1016/j.jhydrol.2013.10.040
He X, Li P, Wu J, Wei M, Ren X, Wang D (2020) Poor groundwater quality and high potential health risks in the Datong Basin, northern China: research from published data. Environ Geochem Health. https://doi.org/10.1007/s10653-020-00520-7
Jacks G, Sharma VP (1983) Nitrogen circulation and nitrate in groundwater in an agricultural catchment in southern India. Environ Geol 5:61–64. https://doi.org/10.1007/BF02381097
Jia H, Howard K, Qian H (2020) Use of multiple isotopic and chemical tracers to identify sources of nitrate in shallow groundwaters along the northern slope of the Qinling Mountains, China. Appl Geochem 113. https://doi.org/10.1016/j.apgeochem.2019.104512
Karunanidhi D, Aravinthasamy P, Subramani T, Wu J, Srinivasamoorthy K (2019) Potential health risk assessment for fluoride and nitrate contamination in hard rock aquifers of Shanmuganadhi River basin, South India. Hum Ecol Risk Assess Int J 1–21. https://doi.org/10.1080/10807039.2019.1568859
Kaviarasan M, Geetha P, Soman KP (2016) GIS-based ground water quality monitoring in Thiruvannamalai district, Tamil Nadu, India. In: LP Suresh, BK Panigrahi (eds) Proceedings of the International Conference on Soft Computing Systems, New Delhi. Springer India, pp 685–700
Li EX, Ling B (2006) Effect of water pollution on human health. Sanit Eng China 5(1):3–5
Li X, Zhang L, Hou X (2008) Use of hydrogeochemistry and environmental isotopes for evaluation of groundwater in Qingshuihe Basin, northwestern China. Hydrogeol J 16:335–348. https://doi.org/10.1007/s10040-007-0269-7
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71:3133–3145. https://doi.org/10.1007/s12665-013-2691-6
Li P, He X, Guo W (2019) Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: a case study in Yan’an city on the loess plateau of Northwest China. Hum Ecol Risk Assess 25:11–31. https://doi.org/10.1080/10807039.2018.1553612
Lockhart KM, King AM, Harter T (2013) Identifying sources of groundwater nitrate contamination in a large alluvial groundwater basin with highly diversified intensive agricultural production. J Contam Hydrol 151:140–154. https://doi.org/10.1016/j.jconhyd.2013.05.008
Mohebbi MR, Saeedi R, Montazeri A, Azam Vaghefi K, Labbafi S, Oktaie S, Abtahi M, Mohagheghian A (2013) Assessment of water quality in groundwater resources of Iran using a modified drinking water quality index (DWQI). Ecol Indic 30:28–34. https://doi.org/10.1016/j.ecolind.2013.02.008
Nalbantcilar MT, Pinarkara SY (2016) Public health risk assessment of groundwater contamination in Batman, Turkey. J Water Health 14:650–661. https://doi.org/10.2166/wh.2016.290
NEERI (1991) Water mission report on participation of NEERI. NEERI (National Environmental Engineering Research Institute), Nagpur
Panno SV, Hackley KC, Hwang HH, Greenberg SE, Krapac IG, Landsberger S, O'Kelly DJ (2006) Characterization and identification of Na–Cl sources in ground water. Ground Water 44:176–187. https://doi.org/10.1111/j.1745-6584.2005.00127.x
Papazotos P, Vasileiou E, Perraki M (2019) The synergistic role of agricultural activities in groundwater quality in ultramafic environments: the case of the Psachna basin, central Euboea, Greece. Environ Monit Assess 191(5):317. https://doi.org/10.1007/s10661-019-7430-3
Saha P, Paul B (2019) Groundwater quality assessment in an industrial hotspot through interdisciplinary techniques. Environ Monit Assess 191(S2). https://doi.org/10.1007/s10661-019-7418-z
Sajil Kumar PJ (2014) Evolution of groundwater chemistry in and around Vaniyambadi industrial area: differentiating the natural and anthropogenic sources of contamination. Geochemistry 74(4):641–651. https://doi.org/10.1016/j.chemer.2014.02.002
Sajil Kumar PJ, Jegathambal P, James EJ (2014) Chemometric evaluation of nitrate contamination in the groundwater of a hard rock area in Dharapuram, South India. Appl Water Sci 4(4):397–405. https://doi.org/10.1007/s13201-014-0155-0
Sajil Kumar PJ, Anju AM, Vicky E (2019) Hydrogeochemical analysis of groundwater in Thanjavur district, Tamil Nadu; influences of geological settings and land use pattern. Geol Ecol Landsc 1–12. https://doi.org/10.1080/24749508.2019.1695713
Sawyer CN (1967) Chemistry for sanitary engineers. McGraw-Hill, New York
Şener Ş, Şener E, Davraz A (2017) Assessment of groundwater quality and health risk in drinking water basin using GIS. J Water Health 15(1):112–132. https://doi.org/10.2166/wh.2016.148
Shukla S, Saxena A (2018) Global status of nitrate contamination in groundwater: its occurrence, health impacts, and mitigation measures. In: Hussain CM (ed) Handbook of environmental materials management. Springer International Publishing, Cham, p 1–21
SPA (2008) Coimbatore regional plan-2038 volume:1 Tiruppur sub region. School of Planning and Architecture, Bhopal, p 257
Spalding RF, Exner ME (1993) Occurrence of nitrate in groundwater—a review. J Environ Qual 22(3):392–402. https://doi.org/10.2134/jeq1993.00472425002200030002x
Steube C, Richter S, Griebler C (2009) First attempts towards an integrative concept for the ecological assessment of groundwater ecosystems. Hydrogeol J 17(1):23–35. https://doi.org/10.1007/s10040-008-0346-6
Suthar S, Bishnoi P, Singh S, Mutiyar PK, Nema AK, Patil NS (2009) Nitrate contamination in groundwater of some rural areas of Rajasthan, Indiagerd. J Hazard Mater. 171(1–3):189–199. https://doi.org/10.1016/j.jhazmat.2009.05.111
Taneja P, Labhasetwar P, Nagarnaik P (2017) Nitrate in drinking water and vegetables: intake and risk assessment in rural and urban areas of Nagpur and Bhandara districts of India. Environ Sci Pollut Res 26:2026–2037. https://doi.org/10.1007/s11356-017-9195-y
Taufiq A, Effendi AJ, Iskandar I, Hosono T, Hutasoit LM (2019) Controlling factors and driving mechanisms of nitrate contamination in groundwater system of Bandung Basin, Indonesia, deduced by combined use of stable isotope ratios, CFC age dating, and socioeconomic parameters. Water Res 148:292–305. https://doi.org/10.1016/j.watres.2018.10.049
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New Yorl
U.S. EPA. 2014. Human health evaluation manual, supplemental guidance: Update of standard default exposure factors-OSWER directive 9200.1–120. PP.6
Vanhatalo A, Blackwell JR, L’Heureux JE, Williams DW, Smith A, van der Giezen M, Winyard PG, Kelly J, Jones AM (2018) Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med 124:21–30. https://doi.org/10.1016/j.freeradbiomed.2018.05.078
Wakida FT, Lerner DN (2005) Non-agricultural sources of groundwater nitrate: a review and case study. Water Res 39(1):3–16. https://doi.org/10.1016/j.watres.2004.07.026
Wang S, Zheng W, Currell M, Yang Y, Zhao H, Lv M (2017) Relationship between land-use and sources and fate of nitrate in groundwater in a typical recharge area of the North China Plain. Sci Total Environ 609:607–620. https://doi.org/10.1016/j.scitotenv.2017.07.176
WHO (2011) Guidelines for drinking-water quality. World Health Organization, Geneva
WHO (2017) Guidelines for drinking-water quality: fourth edition incorporating the first addendum. World Health Organization, Geneva
Zendehbad M, Cepuder P, Loiskandl W, Stumpp C (2019) Source identification of nitrate contamination in the urban aquifer of Mashhad, Iran. J Hydrol Reg Stud 25:100618. https://doi.org/10.1016/j.ejrh.2019.100618
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: PJ Sajil Kumar; methodology: PJ Sajil Kumar; formal analysis and investigation: PJ Sajil Kumar, Lemoon Kuriachan; writing—original draft preparation: PJ Sajil Kumar, Lemoon Kuriachan; writing—review and editing: PJ Sajil Kumar, Lemoon Kuriachan; funding acquisition: NA; resources: PJ Sajil Kumar, Lemoon Kuriachan; Supervision: PJ Sajil Kumar. Lemoon Kuriachan is the co-author.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Responsible editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pazhuparambil Jayarajan, S.K., Kuriachan, L. Exposure and health risk assessment of nitrate contamination in groundwater in Coimbatore and Tirupur districts in Tamil Nadu, South India. Environ Sci Pollut Res 28, 10248–10261 (2021). https://doi.org/10.1007/s11356-020-11552-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11552-y