Abstract
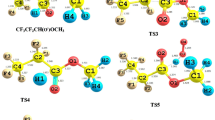
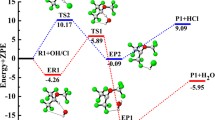
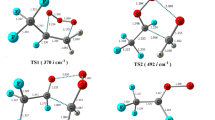
To understand the atmospheric chemistry of hydrofluoroethers, we have studied the oxidation of a highly fluorinated compound n-C2F5CF(OCH3)CF(CF3)2 (HFE-7300) by OH/Cl oxidants. Here, we have employed M06-2X functional along with a 6-31 + G(d,p) basis set to obtain the optimized structures, various forms of energies, and different modes of frequencies for all species. We have characterized energies of all species on the potential energy surface, and it indicates that H-abstraction from n-C2F5CF(OCH3)CF(CF3)2 by Cl atom is kinetically more dominant than the H-abstraction reaction initiated by OH radical. In contrast, the calculated energy change (ΔrH°298 and ΔrG°298) results govern that OH-initiated H-abstraction reaction is highly exothermic and spontaneous compared to the Cl-initiated H-abstraction reaction. Rate constants are estimated using transition state theory as well as canonical variation transition state theory at the temperature range 200–1000 K and 1 atm pressure. The calculated rate constants of the H-abstraction channels are found to be in good agreement with the reported experimental rate constant at 298 K. Moreover, we have estimated the atmospheric lifetimes of HFE-7300 for the reaction with OH radical and Cl atom and are found to be 1.75 and 153.93 years, respectively. Additionally, the global warming potentials for HFE-7300 molecule are also estimated for 20-, 100-, and 500-year time horizons. Further, subsequent aerial oxidation of product radical (n-C2F5CF(OCH2)CF(CF3)2) in the presence of NO radical is performed, and it produced alkoxy radical via formation of peroxy radical. This alkoxy radical undergoes unimolecular decompositions via two different ways and formed n-C2F5CF(OCHO)CF(CF3)2 and n-C2F5CF(OH) CF(CF3)2 products.






Similar content being viewed by others
References
Bivens DB, Minor BH (1998) Fluoroethers and other next generation fluids. Int J Refrig 21:567–576
Blowers P, Moline DM, Tetrault KF, Wheeler RNR, Tuchawena SL (2008) Global warming potentials of hydrofluoroethers. Environ Sci Technol 42:1301–1307
Bravo I, Aranda A, Hurley MD, Marston G, Nutt DR, Shine KP, Smith K, Wallington TJ (2010) Infrared absorption spectra, radiative efficiencies, and global warming potentials of perfluorocarbons: Comparison between experiment and theory. J Geophys Res-Atmos 115:D24317 (1-12)
Bravo I, Díaz-de-Mera Y, Aranda A, Moreno E, Nutt DR, Marston G (2011a) Radiative efficiencies for fluorinated esters: indirect global warming potentials of hydrofluoroethers. Phys Chem Chem Phys 13:17185–17193
Bravo I, Marston G, Nutt DR, Shine KP (2011b) Radiative efficiencies and global warming potentials using theoretically determined absorption cross-sections for several hydrofluoroethers (HFEs) and hydrofluoropolyethers (HFPEs). J Quant Spectrosc Radiat Transf 112:1967–1977
Canneaux S, Bohr F, Henon E (2014) KiSThelP: A program to predict thermodynamic properties and rate constants from quantum chemistry results. J Comput Chem 35:82–93
Christensen LK, Sehested J, Nielsen OJ, Bilde M, Wallington TJ, Guschin A, Molina LT, Molina MJ (1998) Atmospheric chemistry of HFE-7200 (C4F9OC2H5): reaction with OH radicals and fate of C4F9OCH2CH2O• and C4F9OCHO•CH3 radicals. J Phys Chem A 102:4839–4845
Devotta S, Gopichand S, Pendyala VR (1994) Comparative assessment of some HCFCs, HFCs and HFEs as alternatives to CFC11. Int J Refrig 17:32–39
Díaz-de-Mera Y, Aranda A, Bravo I, Moreno E, Martínez E, Rodríguez A (2009) Atmospheric HFEs degradation in the gas phase: reaction of HFE-7500 with Cl atoms at low temperatures. Chem Phys Lett 479:20–24
Federal Register, 2017, 82 (No.139), 33809 (https://www.govinfo.gov/content/pkg/FR-2017-07-21/pdf/2017-15379.pdf).
Finlayson BJ, Pitts JN Jr (2000) Chemistry of the upper and lower atmosphere: theory, experiments, and application. Academic Press, San Diego
Frisch MJ et al (2009) Gaussian 09. Revision D.01. Gaussian, Inc, Wallingford, CT.
Garrett BC, Truhlar DG, Schatz GC (1986) Test of variational transition state theory and multidimensional semiclassical transmission coefficients methods against accurate quantal rate constants for H+ H2/HD, D+ H2, and O+ H2/D2/HD, including intra-and intermolecular kinetic isotope effects. J Am Chem Soc 108:2876–2881
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Gour NK, Deka RC, Singh HJ, Mishra BK (2014) A computational perspective on mechanism and kinetics of the reactions of CF3C(O)OCH2CF3 with OH radicals and Cl atoms at 298 K. J Fluor Chem 160:64–71
Gour NK, Mishra BK, Sarma PJ, Begum P, Deka RC (2017) Tropospheric degradation of HFE-7500 [n-C3F7CF(OCH2CH3)CF(CF3)2] initiated by OH radicals and fate of alkoxy radical [n-C3F7CF (OCH (O)CH3)CF(CF3) 2]: a DFT investigation. J Fluor Chem 204:11–17
Gour NK, Borthakur K, Paul S, Deka RC (2019) Tropospheric degradation of 2-fluoropropene (CH3CF=CH2) initiated by hydroxyl radical: reaction mechanisms, kinetics and atmospheric implications from DFT study. Chemosphere 238:124556
Hammitt JK, Jain AK, Adams JL, Wuebbles DJ (1996) A welfare-based index for assessing environmental effects of greenhouse-gas emissions. Nature 381:301–303
Hammond GS (1955) A correlation of reaction rates. J Am Chem Soc 77:334–338
Hashemi SR, Saheb V, Hosseini SMA (2016) Theoretical studies on the mechanism and kinetics of the hydrogen abstraction reactions from C4F9OC2H5 (HFE-7200) by OH and Cl radicals. J Fluor Chem 187:9–14
Hein R, Crutzen PJ, Heimann M (1997) An inverse modeling approach to investigate the global atmospheric methane cycle. Glob Biogeochem Cycles 11:43–76
Hodnebrog Ø, Etminan M, Fuglestvedt JS, Marston G, Myhre G, Nielsen CJ, Shine KP, Wallington TJ (2013) Global warming potentials and radiative efficiencies of halocarbons and related compounds: a comprehensive review. Rev Geophys 51:300–378
Johnston HS, Heicklen J (1962) Tunnelling corrections for unsymmetrical Eckart potential energy barriers. J Phys Chem 66:532–533
Jordan A, Frank H (1999) Trifluoroacetate in the environment. Environ Sci Technol 33:522–527
Kurylo MJ, Orkin VL (2003) Determination of atmospheric lifetimes via the measurement of OH radical kinetics. Chem Rev 103:5049–5076
Laidler KJ (2004) Chemical kinetics, 3rd edn. Pearson Education, Delhi
Mishra BK, Gour NK, Bhattacharjee D, Deka RC (2016) Atmospheric chemistry of HFE-7000 (i-C3F7OCH3) and isofluoro-propyl formate (i-C3F7OC(O) H): reactions with OH radicals, atmospheric lifetime and fate of alkoxy radical (i-C3F7OCH2O•)–a DFT study. Mol Phys 114:618–626
M™ Novec™ 7300 Engineered Fluid (n.d.) Product Information (https://multimedia.3m.com/mws/media/338713O/3m-novec-7300-engineered-fluid.pdf)
Ninomiya Y, Kawasaki M, Guschin A, Molina LT, Molina MJ, Wallington TJ (2000) Atmospheric chemistry of n-C3F7OCH3: reaction with OH radicals and Cl atoms and atmospheric fate of n-C3F7OCH2O· radicals. Environ Sci Technol 34:2973–2978
Orlando JJ, Tyndall GS, Wallington TJ (2003) The atmospheric chemistry of alkoxy radicals. Chem Rev 103:4657–4690
Oyaro N, Sellevåg SR, Nielsen CJ (2004) Study of the OH and Cl-initiated oxidation, IR absorption cross-section, radiative forcing, and global warming potential of four C4-hydrofluoroethers. Environ Sci Technol 38:5567–5576
Papadimitriou VC, Kambanis KG, Lazarou YG, Papagiannakopoulos P (2004) Kinetic study for the reactions of several hydrofluoroethers with chlorine atoms. J Phys Chem A 108:2666–2674
Paul S, Deka RC, Gour NK (2018) Kinetics, mechanism, and global warming potentials of HFO-1234yf initiated by O3 molecules and NO3 radicals: insights from quantum study. Environ Sci Pollut Res 25:26144–26156
Paul S, Gour NK, Deka RC (2019) Mechanistic investigation of the atmospheric oxidation of bis (2-chloroethyl) ether (ClCH2CH2OCH2CH2Cl) by OH and NO3 radicals and Cl atoms: a DFT approach. J Mol Model 25:43 (1-9)
Pinnock S, Hurley MD, Shine KP, Wallington TJ, Smyth TJ (1995) Radiative forcing of climate by hydrochlorofluorocarbons and hydrofluorocarbons. J Geophys Res-Atmos 100:23227–23238
Ponnusamy S, Sandhiya L, Senthilkumar K (2018) Atmospheric oxidation mechanism and kinetics of hydrofluoroethers, CH3OCF3, CH3OCHF2, and CHF2OCH2CF3, by OH radical: a theoretical study. J Phys Chem A 122:4972–4982
Rao PK, Deka RC, Gour NK, Gejji SP (2018) Understanding the atmospheric oxidation of HFE-7500 (C3F7CF(OC2H5)CF(CF3)2) initiated by Cl Atom and NO3 radical from theory. J Phys Chem A 122:6799–6808
Rodríguez A, Rodríguez D, Moraleda A, Bravo I, Moreno E, Notario A (2014) Atmospheric chemistry of HFE-7300 and HFE-7500: temperature dependent kinetics, atmospheric lifetimes, infrared spectra and global warming potentials. Atmos Environ 96:145–153
Seikya A, Misaki S (1996) A continuing search for new refrigerants. Chemtech 26:44–48
Sekiya A, Misaki S (2000) The potential of hydrofluoroethers to replace CFCs, HCFCs and PFCs. J Fluor Chem 101:215–221
Singleton DL, Cvetanovic RJ (1976) Temperature dependence of the reaction of oxygen atoms with olefins. J Am Chem Soc 98(22):6812–6819
Spicer CW, Chapman EG, Finlayson-Pitts BJ, Plastridge RA, Hubbe JM, Fast JD, Berkowitz CM (1998) Unexpectedly high concentrations of molecular chlorine in coastal air. Nature 394:353–356
Spivakovsky CM, Logan JA, Montzka SA, Balkanski YJ, Foreman-Fowler M, Jones DBA, Horowitz LW, Fusco AC, Brenninkmeijer CAM, Prather MJ, Wofsy SC (2000) Three-dimensional climatological distribution of tropospheric OH: update and evaluation. J Geophys Res-Atmos 105:8931–8980
Tokuhashi K, Takahashi A, Kaise M, Kondo S, Sekiya A, Yamashita S, Ito H (2000) Rate constants for the reactions of OH radicals with CH3OCF2CHF2, CHF2OCH2CF2CHF2, CHF2OCH2CF2CF3, and CF3CH2OCF2CHF2 over the temperature range 250 − 430 K. J Phys Chem A 104:1165–1170
Truhlar DG, Garrett BC (1984) Variational transition state theory. Annu Rev Phys Chem 35:159–189
Tsai WT (2005) Environmental risk assessment of hydrofluoroethers (HFEs). J Hazard Mater 119:69–78
Vereecken L, Francisco JS (2012) Theoretical studies of atmospheric reaction mechanisms in the troposphere. Chem Soc Rev 41:6259–6293
Wallington TJ, Schneider WF, Sehested J, Bilde M, Platz J, Nielsen OJ, Christensen LK, Molina MJ, Molina LT, Wooldridge PW (1997) Atmospheric chemistry of HFE-7100 (C4F9OCH3): reaction with OH radicals, UV spectra and kinetic data for C4F9OCH2· and C4F9OCH2O2· radicals, and the atmospheric fate of C4F9OCH2O· radicals. J Phys Chem A 101:8264–8274
Wallington TJ, Hurley MD, Fedotov V, Morrell C, Hancock G (2002) Atmospheric chemistry of CF3CH2OCHF2 and CF3CHClOCHF2: kinetics and mechanisms of reaction with Cl atoms and OH radicals and atmospheric fate of CF3C(O•)HOCHF2 and CF3C(O•)ClOCHF2 radicals. J Phys Chem A 106:8391–8398
Wei B, Sun J, Mei Q, An Z, Wang X, He M (2018) Theoretical study on gas-phase reactions of nitrate radicals with methoxyphenols: mechanism, kinetic and toxicity assessment. Environ Pollut 243:1772–1780
Wingenter OW, Kubo MK, Blake NJ, Smith TW Jr, Blake DR, Rowland FS (1996) Hydrocarbon and halocarbon measurements as photochemical and dynamical indicators of atmospheric hydroxyl, atomic chlorine, and vertical mixing obtained during Lagrangian flights. J Geophys Res-Atmos 101:4331–4340
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Acknowledgments
Dr. SP is thankful to the University Grant Commission (UGC), New Delhi, for providing financial support from Dr. D. S. Kothari Post-Doctoral Fellowship (Award letter no: F.4-2/2006(BSR)/CH/16-17/0152). S. D. Baruah is thankful to the Department of Science and Technology (DST), New Delhi, for providing him INSPIRE fellowship (No.IF160658).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict(s) of interest.
Additional information
Responsible Editor: Philipp Gariguess
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Paul, S., Mishra, B.K., Baruah, S.D. et al. Atmospheric oxidation of HFE-7300 [n-C2F5CF(OCH3)CF(CF3)2] initiated by •OH/Cl oxidants and subsequent degradation of its product radical: a DFT approach. Environ Sci Pollut Res 27, 907–920 (2020). https://doi.org/10.1007/s11356-019-06975-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06975-1