Abstract
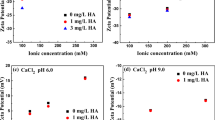
The effects of clay particles (montmorillonite, M) and phosphate (P) on the transport of hydrochar nanoparticles (NPs) in water-saturated porous media (uncoated and aluminum (Al) oxide-coated sands) were explored in NaCl (1–50 mM) solutions. Our results showed that the deposition behaviors of hydrochar NPs affected by M and phosphate were significantly different between pH 6.0 and pH 9.0, especially in Al oxide-coated sand. This can be attributed to their distinct surface characteristics: hydrochar agglomerates with a larger pore size distribution, more carboxylate groups, and less negative charges on the surface at pH 9.0 than those at pH 6.0. In Al oxide-coated sand, block adsorption of hydrochar was alleviated appreciably with the presence of M due to the preferential preoccupies of M on these favorable retention sites. On the contrary, M substantially increased the hydrochar retention on uncoated sand due to the formation of nanoaggregates between hydrochar and M. Differently, phosphate substantially enhanced the transport of hydrochar, even in coated sand, due to the strong phosphate adsorption onto Al oxide on the surface of sand and hydrochar. Our findings will provide useful insights into designing effective strategies for land application of hydrochar while minimizing potential environmental risks.

Graphical abstract








Similar content being viewed by others
References
Abel S, Peters A, Trinks S, Schonsky H, Facklam M, Wessolek G (2013) Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 202-203(2):183–191
Berge ND, Ro KS, Mao J, Flora JR, Chappell MA, Bae S (2011) Hydrothermal carbonization of municipal waste streams. Environ Sci Technol 45(13):5696–5703
Cai L, Tong M, Wang X, Kim H (2014) Influence of clay particles on the transport and retention of titanium dioxide nanoparticles in quartz sand. Environ Sci Technol 48(13):7323–7332
Chen KL, Elimelech M (2006) Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir 22(26):10994–11001
Chen G, Liu X, Su C (2011) Transport and retention of TiO2 rutile nanoparticles in saturated porous media under low-ionic-strength conditions: measurements and mechanisms. Langmuir 27(9):5393–5402
Chen M, Xu N, Cao X, Zhou K, Chen Z, Wang Y, Liu C (2015) Facilitated transport of anatase titanium dioxides nanoparticles in the presence of phosphate in saturated sands. J Colloid Interface Sci 451:134–143
Chen M, Wang D, Yang F, Xu X, Xu N, Cao X (2017) Transport and retention of biochar nanoparticles in a paddy soil under environmentally-relevant solution chemistry conditions. Environ Pollut 230:540–549
Chen M, Alim N, Zhang Y, Xu N, Cao X (2018) Contrasting effects of biochar nanoparticles on the retention and transport of phosphorus in acidic and alkaline soils. Environ Pollut 239:562–570
Chen M, Tao X, Wang D, Xu Z, Xu X, Hu X, Xu N, Cao X (2019) Facilitated transport of cadmium by biochar-Fe3O4 nanocomposites in water-saturated natural soils. Sci Total Environ 684:265–275
Chowdhury I, Hong Y, Honda RJ, Walker SL (2011) Mechanisms of TiO2 nanoparticle transport in porous media: role of solution chemistry, nanoparticle concentration, and flowrate. J Colloid Interface Sci 360(2):548–555
Chowdhury I, Cwiertny DM, Walker SL (2012) Combined factors influencing the aggregation and deposition of nano-TiO2 in the presence of humic acid and bacteria. Environ Sci Technol 46(13):6968–6976
Cui HJ, Wang MK, Fu ML, Ci E (2011) Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed on ferrihydrite using rice straw-derived biochar. J Soils Sediments 11(7):1135–1141
Fang J, Shan X, Wen B, Huang R (2013a) Mobility of TX100 suspended multiwalled carbon nanotubes (MWCNTs) and the facilitated transport of phenanthrene in real soil columns. Geoderma 207:1–7
Fang J, Xu MJ, Wang DJ, Wen B, Han JY (2013b) Modeling the transport of TiO2 nanoparticle aggregates in saturated and unsaturated granular media: effects of ionic strength and pH. Water Res 47(3):1399–1408
Fang J, Gao B, Chen J, Zimmerman AR (2015) Hydrochars derived from plant biomass under various conditions: characterization and potential applications and impacts. Chem Eng J 267:253–259
Fang J, Wang M, Shen B, Zhang L, Lin D (2017) Distinguishable co-transport mechanisms of phenanthrene and oxytetracycline with oxidized-multiwalled carbon nanotubes through saturated soil and sediment columns: vehicle and competition effects. Water Res 108:271–279
Fang J, Zhan L, Ok YS, Gao B (2018) Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J Ind Eng Chem 57:15–21
Flora JFR, Lu X, Li L, Flora JRV, Berge ND (2013) The effects of alkalinity and acidity of process water and hydrochar washing on the adsorption of atrazine on hydrothermally produced hydrochar. Chemosphere 93(9):1989–1996
Gajic A, Koch HJ (2012) Sugar beet (l.) growth reduction caused by hydrochar is related to nitrogen supply. J Environ Qual 41(4):1067–1075
George C, Wagner M, Kucke M, Rillig MC (2012) Divergent consequences of hydrochar in the plant-soil system: arbuscular mycorrhiza, nodulation, plant growth and soil aggregation effects. Appl Soil Ecol:68–72
Gu X, Guo Y, Zhang C, Ding L, Han X, Liu Q, Zou B (2016) The effect of phenolic compounds on the preparation of hydrochars from saccharides. Environ Prog Sustain Energy 35(1):189–194
Guo P, Xu N, Li D, Huangfu X, Li Z (2018) Aggregation and transport of rutile titanium dioxide nanoparticles with montmorillonite and diatomite in the presence of phosphate in porous sand. Chemosphere 204:327–334
He J, Li C, Wang D, Zhou D (2015) Biofilms and extracellular polymeric substances mediate the transport of graphene oxide nanoparticles in saturated porous media. J Hazard Mater 300:467–474
Hu B, Wang K, Wu L, Yu S, Antonietti M, Titirici MM (2010) Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv Mater 22(7):813–828
Huangfu X, Xu N, Yang J, Yang H, Zhang M, Ye Z, Wang S, Chen J (2019) Transport and retention of hydrochar-diatomite nanoaggregates in water-saturated porous sand: effect of montmorillonite and phosphate at different ionic strengths and solution pH. Sci Total Environ 703:134487–134487
Ibarra J, Munoz E, Moliner R (1996) FTIR study of the evolution of coal structure during the coalification process. Org Geochem 24(6):725–735
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sust Energ Rev 45:359–378
Kasel D, Bradford SA, Simunek J, Heggen M, Vereecken H, Klumpp E (2013) Transport and retention of multi-walled carbon nanotubes in saturated porous media: effects of input concentration and grain size. Water Res 47(2):933–944
Kuan WH, Lo SL, Wang MK, Lin CF (1998) Removal of Se (IV) and Se (VI) from water by aluminum-oxide-coated sand. Water Res 32(3):915–923
Liu Z, Zhang F, Wu J (2010) Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 89(2):510–514
Liu C, Xu N, Feng G, Zhou D, Cheng X, Li Z (2017) Hydrochars and phosphate enhancing the transport of nanoparticle silica in saturated sands. Chemosphere 189:213–223
Liu K, Ostadhassan M, Sun L, Zouc J, Yuan Y, Gentzis T, Zhang Y, Carvajal-Ortiz H, Rezaee R (2019) A comprehensive pore structure study of the Bakken Shale with SANS, N2 adsorption and mercury intrusion. Fuel 245:274–285
Lohwacharin J, Takizawa S, Punyapalakul R (2015) Carbon black retention in saturated natural soils: Effects of flow conditions, soil surface roughness and soil organic matter. Environ Pollut 205:131–138
Parks GA (1965) The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem Rev 65(2):177–198
Phenrat T, Kim HJ, Fagerlund F, Illangasekare T, Lowry GV (2009) Particle size distribution, concentration, and magnetic attraction affect transport of polymer-modified Fe(0) nanoparticles in sand columns. Environ Sci Technol 43(13):5079–5085
Pradhan BK, Sandle NK (1999) Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon 37(8):1323–1332
Sevilla M, Fuertes AB (2009) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47(9):2281–2289
Shellenberger K, Logan BE (2002) Effect of molecular scale roughness of glass beads on colloidal and bacterial deposition. Environ Sci Technol 36(2):184–189
Stemann J, Putschew A, Ziegler F (2013) Hydrothermal carbonization: process water characterization and effects of water recirculation. Bioresour Technol 143:139–146
Sun Y, Yang G, Wen C, Zhang L, Wang YS (2014) Preparation of carbon sphere from lactose by hydrothermal reaction and its performance in gas separation. Environ Prog Sustain Energy 33(2):581–587
Sun P, Shijirbaatar A, Fang J, Owens G, Lin D, Zhang K (2015) Distinguishable transport behavior of zinc oxide nanoparticles in silica sand and soil columns. Sci Total Environ 505(505):189–198
Takaya CA, Fletcher LA, Singh S, Anyikude KU, Ross AB (2016) Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 145:518–527
Teo J, Teo L, Liew WK, Leong YK (2009) Clay, phosphate adsorption, dispersion, and rheology. Water Air Soil Pollut Focus 9:403–407
Tombacz E, Szekeres M (2006) Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl Clay Sci 34:105–124
Tong M, Ding J, Shen Y, Zhu P (2010) Influence of biofilm on the transport of fullerene (C60) nanoparticles in porous media. Water Res 44(4):1094–1103
Wang L, Guo Y, Zhu Y, Li Y, Qu Y, Rong C, Wang Z (2010a) A new route for preparation of hydrochars from rice husk. Bioresour Technol 101(24):9807–9810
Wang Y, Li Y, Kim H, Walker SL, Abriola LM, Pennell KD (2010b) Transport and retention of fullerene nanoparticles in natural soils. J Environ Qual 39(6):1925–1933
Wang D, Bradford SA, Harvey RW, Gao B, Cang L, Zhou D (2012a) Humic acid facilitates the transport of ARS-labeled hydroxyapatite nanoparticles in iron oxyhydroxide-coated sand. Environ Sci Technol 46(5):2738–2745
Wang D, Bradford SA, Harvey RW, Hao X, Zhou D (2012b) Transport of ARS-labeled hydroxyapatite nanoparticles in saturated granular media is influenced by surface charge variability even in the presence of humic acid. J Hazard Mater:170–176
Wang D, Zhang W, Hao X, Zhou D (2013a) Transport of biochar particles in saturated granular media: effects of pyrolysis temperature and particle size. Environ Sci Technol 47(2):821–828
Wang D, Zhang W, Zhou D (2013b) Antagonistic effects of humic acid and iron oxyhydroxide grain-coating on biochar nanoparticle transport in saturated sand. Environ Sci Technol 47:5154–5161
Wang D, Shen C, Jin Y, Su C, Zhou D (2017) Role of solution chemistry in the retention and release of graphene oxide nanomaterials in uncoated and iron oxide-coated sand. Sci Total Environ 579:776–785
Wang Y, Zhang W, Shang J, Shen C, Joseph SD (2019) Chemical aging changed aggregation kinetics and transport of biochar colloids. Environ Sci Technol 53:8136–8146
Xu X, Xu N, Cheng X, Guo P, Chen Z, Wang D (2017) Transport and aggregation of rutile titanium dioxide nanoparticles in saturated porous media in the presence of ammonium. Chemosphere 169:9–17
Xu N, Cheng X, Zhou K, Xu X, Li Z, Chen J, Li D (2018) Facilitated transport of titanium dioxide nanoparticles via hydrochars in the presence of ammonium in saturated sands: Effects of pH, ionic strength, and ionic composition. Sci Total Environ 612:1348–1357
Xu N, Huangfu X, Li Z, Wu Z, Li D, Zhang M (2019) Nanoaggregates of silica with kaolinite and montmorillonite: sedimentation and transport. Sci Total Environ 669:893–902
Yao Y, Gao B, Chen J, Yang L (2013) Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ Sci Technol 47(15):8700–8708
Zhang W, Niu J, Morales VL, Chen X, Hay AG, Lehmann J, Steenhuis TS (2010) Transport and retention of biochar particles in porous media: effect of pH, ionic strength, and particle size. Ecohydrology 3(4):497–508
Zhang J, Lin Q, Zhao X (2014) The hydrochar characters of municipal sewage sludge under different hydrothermal temperatures and durations. J Integr Agric 13(3):471–482
Zhou D, Abdel-Fattah AI, Keller AA (2012) Clay particles destabilize engineered nanoparticles in aqueous environments. Environ Sci Technol 46(14):7520–7526
Funding
The authors appreciate the research funding provided by the National Natural Science Foundation of China (21777110 and 21377090) and the Jiangsu Collaborative Innovation Center of Technology and Material for Water Treatment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary materials
ESM 1
(DOC 1350 kb)
Rights and permissions
About this article
Cite this article
Yang, J., Chen, M., Yang, H. et al. Surface heterogeneity mediated transport of hydrochar nanoparticles in heterogeneous porous media. Environ Sci Pollut Res 27, 32842–32855 (2020). https://doi.org/10.1007/s11356-020-09482-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09482-w