Abstract
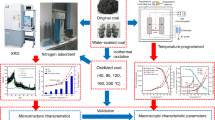
In order to study the effects of chemical composite additive (CCA) on the microscopic characteristics of spontaneous combustion coal, atomic force microscope and Fourier transform infrared spectroscopy technology were used to study the microstructure and active groups of spontaneous combustion coal. The roughness, three-dimensional surface morphology, microscopic pore structure, infrared spectrum, and active group content of raw coal samples and coal samples treated with water or different concentrations of CCA were analyzed. The experimental results showed that compared with the raw coal, the roughness Rq and Ra of the CCA-treated coal samples decreased with increasing CCA concentration, and the surface topography of the microscopic structure tended to be flat and smooth, and the size becomes smaller and the depth becomes shallow of pore. In the raw coal samples and coal samples treated with water and CCA, the main types of active groups remained constant. However, the contents of these groups changed, and the order of the contents of main types of active groups is water-treated > raw coal (untreated) > CCA-1% treated > CCA-5% treated > CCA-10% treated > CCA-20% treated. In addition, the mechanism of the CCA inhibition of coal spontaneous combustion was discussed and analyzed.
















Similar content being viewed by others
References
Baris K, Kizgut S, Didari V (2012) Low-temperature oxidation of some Turkish coals. Fuel 93:423–432
Bruening F, Cohen A (2005) Measuring surface properties and oxidation of coal macerals using the atomic force microscope. Int J Coal Geol 63:195–204
Carras J, Day S, Saghafi A, Williams D (2009) Greenhouse gas emissions from low temperature oxidation and spontaneous combustion at open-cut coal mines in Australia. Int J Coal Geol 78:161–168
Colaizzi G (2004) Prevention, control and/or extinguishment of coal seam fires using cellular grout. Int J Coal Geol 59:75–81
Cui C, Jiang S, Zhang W (2017) Experimental study on the effect of thermo-responsive secundine inhibitor on coal spontaneous combustion. Energ Fuel 31:14262–14269
Cummings J, Shah K, Atkin R, Moghtaderi B (2015) Physicochemical interactions of ionic liquids with coal; the viability of ionic liquids for pre-treatments in coal liquefaction. Fuel 143:244–252
Cummings J, Tremain P, Shah K, Heldt E, Moghtaderi B, Atkin R, Kundu S, Vuthaluru H (2017) Modification of lignites via low temperature ionic liquid treatment. Fuel Process Technol 155:51–58
Deng J, Bai Z, Xiao Y, Shu C (2018) Effects on the activities of coal microstructure and oxidation treated by imidazolium-based ionic liquids. J Therm Anal Calorim 133:453–463
Dou G, Wang D, Zhong X, Qin B (2014) Effectiveness of catechin and poly(ethylene glycol) at inhibiting the spontaneous combustion of coal. Fuel Process Technol 120:123–127
Gadelmawla E, Koura M, Maksoud T, Elewa I, Soliman H (2002) Roughness parameters. J Mater Process Technol 123:133–145
Kim A (2004) Cryogenic injection to control a coal waste bank fire. Int J Coal Geol 59:63–73
Kong B, Li Z, Yang Y, Liu Z, Yan D (2017) A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ Sci Pollut R 24:23453–23470
Kuenzer C, Stracher G (2012) Geomorphology of coal seam fires. Geomorphology 138:209–222
Li Z, Kong B, Wei A, Yang Y, Zhou Y, Zhang L (2016) Free radical reaction characteristics of coal low-temperature oxidation and its inhibition method. Environ Sci Pollut R 23:23593–23605
Li J, Li Z, Yang Y, Kong B, Wang C (2018) Laboratory study on the inhibitory effect of free radical scavenger on coal spontaneous combustion. Fuel Process Technol 171:350–360
Monnet A, Percebois J, Gabriel S (2015) Assessing the potential production of uranium from coal-ash milling in the long term. Resour Policy 45:173–182
Painter P, Pulati N, Cetiner R, Sobkowiak M, Mitchell G, Mathews J (2010) Dissolution and dispersion of coal in ionic liquids. Energ Fuel 24:1848–1853
Pan R, Chen L, Li Z, Yu M (2017) The characteristic of micro-fracture evolution and gas permeability effect in the different bedding structure of coal. J Nanosci Nanotechno 17:6670–6676
Pan R, Li C, Fu D, Chen L, Xiao Z (2019) Micromechanism of spontaneous combustion and oxidation of an unloaded coal under repeated disturbance. Int J Energ Res 43:1303–1311
Pandey J, Mohalik N, Mishra R, Khalkho A, Kumar D, Singh V (2015) Investigation of the role of fire retardants in preventing spontaneous heating of coal and controlling coal mine fires. Fire Technol 51:227–245
Pulati N, Sobkowiak M, Mathews J, Painter P (2012) Low-temperature treatment of Illinois no. 6 coal in ionic liquids. Energ Fuel 26:3548–3552
Qin B, Dou G, Wang Y, Xin H, Ma L, Wang D (2017a) A superabsorbent hydrogel–ascorbic acid composite inhibitor for the suppression of coal oxidation. Fuel 190:129–135
Qin B, Ma D, Li F, Li Y (2017b) Aqueous clay suspensions stabilized by alginate fluid gels for coal spontaneous combustion prevention and control. Environ Sci Pollut R 24:24657–24665
Slovák V, Taraba B (2012) Urea and CaCl2 as inhibitors of coal low-temperature oxidation. J Therm Anal Calorim 110:363–367
Song Z, Kuenzer C (2014) Coal fires in China over the last decade: a comprehensive review. Int J Coal Geol 133:72–99
Tahmasebi A, Yu J, Han Y, Li X (2012) A study of chemical structure changes of Chinese lignite during fluidized-bed drying in nitrogen and air. Fuel Process Technol 101:85–93
Tang Y (2018) Experimental investigation of applying MgCl2 and phosphates to synergistically inhibit the spontaneous combustion of coal. J Energy Inst 91:639–645
Taraba B, Peter R, Slovák V (2011) Calorimetric investigation of chemical additives affecting oxidation of coal at low temperatures. Fuel Process Technol 92:712–715
To T, Shah K, Tremain P, Simmons B, Moghtaderi B, Atkin R (2017) Treatment of lignite and thermal coal with low cost amino acid based ionic liquid-water mixtures. Fuel 202:296–306
Tsai Y, Yang Y, Wang C, Shu C, Deng J (2018) Comparison of the inhibition mechanisms of five types of inhibitors on spontaneous coal combustion. Int J Energ Res 42:1158–1171
Wang S, Elsworth D, Liu J (2011) Permeability evolution in fractured coal: the roles of fracture geometry and water-content. Int J Coal Geol 87:13–25
Wang L, Xu Y, Jiang S, Yu M, Chu T, Zhang W, Wu Z, Kou L (2012) Imidazolium based ionic liquids affecting functional groups and oxidation properties of bituminous coal. Safety Sci 50:1528–1534
Wang D, Xin H, Qi X, Dou G, Qi G, Ma L (2016a) Reaction pathway of coal oxidation at low temperatures: a model of cyclic chain reactions and kinetic characteristics. Combust Flame 163:447–460
Wang G, Yan G, Zhang X, Du W, Huang Q, Sun L, Zhang X (2016b) Research and development of foamed gel for controlling the spontaneous combustion of coal in coal mine. J Loss Prevent Proc 44:474–486
Wang S, Liu S, Sun Y, Jiang D, Zhang X (2017) Investigation of coal components of Late Permian different ranks bark coal using AFM and micro-FTIR. Fuel 187:51–57
Xi Z, Guo X, Richard Liew J (2018) Investigation of thermoplastic powder synergizing polymorphic foam to inhibit coal oxidation at low temperature. Fuel 226:490–497
Xu Y, Wang D, Wang L, Zhong X, Chu T (2012) Experimental research on inhibition performances of the sand-suspended colloid for coal spontaneous combustion. Safety Sci 50:822–827
Yadav S, Mondal S (2019) A complete review based on various aspects of pulverized coal combustion. Int J Energ Res 43:3134–3165
Yang Y, Li Z, Si L, Hou S, Zhou Y, Qi Q (2017) Consolidation grouting technology for fire prevention in mined-out areas of working face with large inclined angle and its application. Fire Mater 41:700–715
Zhang Z, Liu C, Liu W, Cui Y, Du X, Xu D, Guo H, Deng Y (2017a) Innovative design of coal utilization-a green pathway for direct conversion of coal to electricity through flow fuel cell technology. Appl Energ 200:226–236
Zhang L, Shi B, Qin B, Wu Q, Dao V (2017b) Characteristics of foamed gel for coal spontaneous combustion prevention and control. Combust Sci Technol 189:980–990
Zhang W, Jiang S, Wu Z, Wang K, Shao H, Qin T, Xi X, Tian H (2018) Influence of imidazolium-based ionic liquids on coal oxidation. Fuel 217:529–535
Zheng L, Li G, Wang Y, Zhu X, Pan R, Wang Y (2018) Effect of blockage ratios on the characteristics of methane/air explosion suppressed by BC powder. J Hazard Mater 353:25–33
Ходот B (1966) Coal and gas burst, translated by Song S, Wang Y. China Industry Publishing House, Beijing, pp 27–30
Acknowledgments
The authors wish to thank these organizations for their support. They also wish to thank the readers and editors for their constructive comments and suggestions to improve the manuscript.
Funding
This work was carried out with funding from the National Key R&D Program of China (Grant No. 2018YFC0808100) and National Natural Science Foundation of China (Grant No. 51304070, 51674103).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pan, R., Ma, J., Zheng, L. et al. Experimental study on the effects of chemical composite additive on the microscopic characteristics of spontaneous combustion coal. Environ Sci Pollut Res 27, 5606–5619 (2020). https://doi.org/10.1007/s11356-019-07340-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07340-y