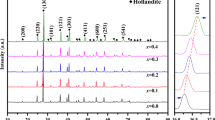
Abstract
This study investigated crystallization mechanisms for the formation of lead aluminosilicate by sintering lead stabilization with kaolin-based precursors. PbAl2Si2O8 was found to be the only stable lead aluminosilicate in low-PbO system and demonstrates its highly intrinsic resistance to acid attack in leaching test. A three-stage PbAl2Si2O8 formation mechanism was supported by the results of the changing temperature in the system. Amorphization of sintered products was observed in both PbO/kaolinite and PbO/mullite systems at 600–700°C. When the temperature was increased to 750–900°C, the crystallochemical formation of lead aluminosilicates (i.e., Pb4Al4Si3O16, Pb6Al6Si2O21, and PbAl2Si2O8) was observed. Pb4Al4Si3O16 and Pb6Al6Si2O21 were found to be the intermediate phases at 700–900°C. Finally, PbAl2Si2O8 was found to be the only crystallite phase to host Pb at above 950°C. A maximum of 80% and 96.7% Pb can be incorporated into PbAl2Si2O8 in PbO/kaolinite and PbO/mullite systems, respectively, but the final products exhibited different microstructures. To reduce environmental hazard of lead, this strategy demonstrated a preferred mechanism of immobilizing lead into PbAl2Si2O8 structure via kaolin-based precursors.









Similar content being viewed by others
References
Al-Degs Y, Khraisheh MAM, Tutunji MF (2001) Sorption of lead ions on diatomite and manganese oxides modified diatomite. Water Res 35(15):3724–3728
Celary P, Szołtysek JS (2014) Vitrification as an alternative to landfilling of tannery sewage sludge. Waste Manag 34(12):2520–2527
Cerbo AAV, Ballesteros F, Chen TC, Lu MC (2017) Solidification/stabilization of fly ash from city refuse incinerator facility and heavy metal sludge with cement additives. Environ Sci Pollut Res 24(2):1748–1756
Champagne CA, Fanguy JC, Heard GE (2000) Solidification/stabilization of lead with the aid of bagasse as an additive to Portland cement. Microchem J 65:255–259
Chen C, Tuan W (2002) Evolution of mullite texture on firing tape-caste kaolin bodies. J Am Ceram Soc 85:1121–1126
Chen S, Zhao B, Hayes PC, Jak E (2001) Experimental study of phase equilibria in the PbO-Al2O3-SiO2 system. Metall Mater Trans B Process Metall Mater Process Sci 32:997–1005
Chen W, Lu Z, Xiao B, Gu P, Yao W, Xing J, Asiri AM, Alamry KA, Wang X, Wang S (2019) Enhanced removal of lead ions from aqueous solution by iron oxide nanomaterials with cobalt and nickel doping. J Clean Prod 211:1250–1258
Conrad K, Hansen HCB (2007) Sorption of zinc and lead on coir. Bioresour Technol 98:89–97
Curetti N, Benna P, Bruno E (2015) High-pressure equation of state and phase transition in PbAl2Si2O8 feldspar. Am Mineral 100:1568–1577
De La Torre AG, Bruque S, Aranda MAG (2001) Rietveld quantitative amorphous content analysis. J Appl Crystallogr 34(2):196–202
Fan HL, Zhou SF, Jiao WZ, Qi GS, Liu YZ (2017) Removal of heavy metal ions by magnetic chitosan nanoparticles prepared continuously via high-gravity reactive precipitation method. Carbohydr Polym 174:1192–1200
Gu P, Zhang S, Zhang C, Wang X, Khan A, Wen T, Hu B, Alsaedi A, Hayat T, Wang X (2019) Two-dimensional MAX-derived titanate nanostructures for efficient removal of Pb (II). Dalton T 48(6):2100–2107
Kukukova A, Aubin J, Kresta SM (2009) A new definition of mixing and segregation: three dimensions of a key process variable. Chem Eng Res Des 87:633–647
Kulkarni VV, Golder AK, Ghosh PK (2018) Critical analysis and valorization potential of battery industry sludge: Speciation, risk assessment and metal recovery. J Clean Prod 171:820–830
Li M, Su P, Guo Y, Zhang W, Mao L (2017) Effects of SiO2, Al2O3 and Fe2O3 on leachability of Zn, cu and Cr in ceramics incorporated with electroplating sludge. J Environ Chem Eng 5(4):3143–3150
Li NH, Chen YH, Hu CY, Hsieh CH, Lo SL (2011a) Stabilization of nickel-laden sludge by a high-temperature NiCr2O4 synthesis process. J Hazard Mater 198:356–361
Li NH, Lo SL, Hu ZY, Hsieh CH, Chen CL (2011b) Stabilization and phase transformation of CuFe2O4 sintered from simulated copper-laden sludge. J Hazard Mater 190(1–3):597–603
Li YC, Min XB, Chai LY, Shi MQ, Tang CJ (2016) Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals. J Environ Manag 181:756–761
Liu XS, Xin B, Dong LY, Liang JS (2018) Composting enhances the removal of lead ions in aqueous solution by spent mushroom substrate: biosorption and precipitation. J Clean Prod 200:1–11
Lu XW, Shih K, Cheng H (2013) Lead glass-ceramics produced from the beneficial use of waterworks sludge. Water Res 47:1353–1360
Lu XW, Ning X, Lee P, Shih K, Wang F (2017) Transformation of hazardous lead into lead ferrite ceramics: crystal structures and their role in lead leaching. J Hazard Mater 336:139–145
Luo S, Xu X, Zhou G, Liu C, Tang Y (2014) Amino siloxane oligomer-linked graphene oxide as an efficient adsorbent for removal of Pb(II; ) from wastewater. J Hazard Mater 274:145–155
MacKenzie KJD, Hartman JS, Okada K (1996) MAS NMR evidence for the presence of silicon in the alumina spinel from thermally transformed kaolinite. J Am Ceram Soc 79:2980–2982
Magallanes-Perdomo M, Carrodeguas RG, Pena P, De Aza PN, De Aza S, De Aza AH (2009) Non-isothermal devitrification study of wollastonite-tricalcium phosphate bioeutectic® glass. Key Eng Mater 396-398:127–130
Mihajlovic ASR, Kremenovic AS, Dosen AM, Andrejic JZ, Dondur VT (2015) Thermally induced phase transformation of Pb-exchanged LTA and FAU-framework zeolite to feldspar phases. Microporous Mesoporous Mater 201:210–218
Paria S, Yuet PK (2006) Solidification-stabilization of organic and inorganic contaminants using Portland cement: a literature review. Environ Rev 14:217–255
Perić J, Trgo M, Vukojević MN (2004) Removal of zinc, copper and lead by natural zeolite-a comparison of adsorption isotherms. Water Res 38:1893–1899
Radovanoviä DÄ, KamberoviÄ ZJ, Koraä MS, Rogan JR (2016) Solidified structure and leaching properties of metallurgical wastewater treatment sludge after solidification/stabilization process. J Environ Sci Health Part A 51(1):34–43
Rendtorff NM, Conconi MS, Aglietti EF, Chain CY, Pasquevich AF, Rivas PC, Martínez JA, Caracoche MC (2010) Phase quantification of mullite-zirconia and zircon commercial powders using PAC and XRD techniques. Hyperfine Interact 198:211–218
Sainz MA, Serrano FJ, Amigo JM, Bastida J, Caballero A (2000) XRD microstructural analysis of mullites obtained from kaolinite–alumina mixtures. J Eur Ceram Soc 20:403–412
Segura SG, Ocon JD, Chong MN (2018) Electrochemical oxidation remediation of real wastewater effluents - a review. Process Saf Environ 113:48–67
Silva KNO, Paiva SSM, Souza FL (2018) Applicability of electrochemical technologies for removing and monitoring Pb2+ from soil and water. J Electroanal Chem 816:171–178
Shih K, White T, Leckie JO (2006) Nickel stabilization efficiency of aluminate and ferrite spinels and their leaching behavior. Environ Sci Technol 40:5520–5526
Stumm W, Morgan J (1996) A Wiley-Interscience publication, 3rd ed by Wiley J and Sons, Inc. Geochim Cosmochim Acta
Su M, Liao C, Chuang KH, Wey MY, Shih K (2015) Cadmium stabilization efficiency and Leachability by CdAl4O7 monoclinic structure. Environ Sci Technol 49:14452–14459
Sun DD, Tay JH, Cheong HK, Leung DLK, QianG (2001) Recovery of heavy metals and stabilization of spent hydrotreating catalyst using a glass-ceramic matrix. J Hazard Mater 87(1–3): 13–223
Tang Y, Liu C, Shih K (2014) Beneficial metal stabilization mechanisms using simulated sludge incineration ash for ceramic products. J Chem Technol Biotechnol 89:536–543
Tang Y, Shih K (2013) Stabilization mechanisms and reaction sequences for sintering simulated copper-laden sludge with alumina. ACS Sustain Chem Eng 1:1239–1245
Tribaudino M, Benna P (1998) Structural variations induced by thermal treatment in lead feldspar (PbAl2Si2O8). Am Mineral 83:159–166
US EPA (1998) Applicability of the Toxicity Characteristic Leaching Procedure to Mineral Processing Wastes <http://www.epa.gov/osw/nonshaz/industrial/special/mining/minedock/tclp/tcremand.pdf>
US EPA (1992) U.S. EPA Method 1311- Toxicity Characteristic Leaching Procedure and its Salts <http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/1311.pdf>
Vedrenne M, Vasquezmedrano R, Pratogarcia D, Frontanauribe BA, Ibanez JG (2012) Characterization and detoxification of a mature landfill leachate using a combined coagulation–flocculation/photo Fenton treatment. J Hazard Mater 205-206:208–215
Venäläunen SH (2011) Apatite ore mine tailings as an amendment for remediation of a lead-contaminated shooting range soil. Sci Total Environ 409:4628–4634
Velde B (1992) Introduction to clay minerals. Chapman & Hall(ed). London J Biosci Bioeng 86:233–235
Reed JS (1995) Principles of ceramic processing, 2nd edn. John Wiley, New York
Wang Y, Shih K, Jiang X (2012) Phase transformation during the sintering of γ-alumina and the simulated Ni-laden waste sludge. Ceram Int 38(3):1879–1886
Wang YS, Dai JG, Wang L, Dcw T, Poon CS (2017) Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 190:90–96
Xu GR, Zou JL, Li GB (2008) Effect of sintering temperature on the characteristics of sludge ceramsite. J Hazard Mater 150:394–400
Xu GR, Zou JL, Li GB (2009) Stabilization/solidification of heavy metals in sludge ceramsite and leachability affected by oxide substances. Environ Sci Technol 43:5902-5907 Zhao D, Yu Y, Chen JP (2016) Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA. Water Res 101:564–573
Yang HC, Yun JS, Kang MJ, Kim JH, Kang Y (1992) Mechanisms and kinetics of cadmium and lead capture by calcined kaolin at high temperatures. Korean J Chem Eng 18:499–505
Yang K, Wu J, His C, Lu H (2011) Morphologically textured mullite in sintered tape-cast kaolin. J Am Ceram Soc 94:938–944
Zhu L, Ji J, Wang S, Xu C, Yang K (2018) Removal of Pb(II) from wastewater using Al2O3-NaA zeolite composite hollow fiber membranes synthesized from solid waste coal fly ash. Chemosphere 206:278
Funding
This research was supported by Science and Technology Program of Guangzhou, China (201804010103), Shaoguan special fund for soil pollution and control (2017sgtyfz302), National Natural Science Foundation of China (Project 21637001), and the Research Fund Program of Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology (2018 K18).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philipp Gariguess
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Lu, X., Liu, Y. et al. Transformation of hazardous lead into aluminosilicate ceramics: structure evolution and lead leaching. Environ Sci Pollut Res 27, 10404–10414 (2020). https://doi.org/10.1007/s11356-019-07153-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07153-z