Abstract
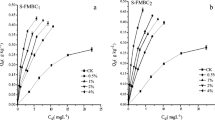
The increasing scarcity of arable land necessitates the development of effective decontamination techniques to re-gain contaminated areas and make them suitable for agricultural and other activities. Herein, we prepare a ferromanganese binary oxide-biochar composite (FMBC) and compare its potential for remediating Cd-contaminated red soil with that of biochar (BC), showing that (i) the obtained adsorption data are well described by the Langmuir model and (ii) Cd adsorption capacity increases with increasing adsorbent dosage. Specifically, the Cd adsorption capacity of FMBC-amended soil (6.72 mg g−1) is demonstrated to significantly exceed that of BC-amended red soil (4.85 mg g−1) and that of the control (2.28 mg g−1) and increases with increasing temperature and pH, while the results of instrumental analyses indicate that Cd sorption on the soil surface occurs via the formation of CdO and Cd(OH)2. Thus, FMBCs are concluded to play an important role in the adsorption of Cd, having the potential to prevent red soil acidification and improve soil quality, and are found to be promising remediation materials for mitigating the risks posed by Cd-contaminated red soil.







Similar content being viewed by others
References
Arias M, Barral MT, Mejuto JC (2002) Enhancement of copper and cadmium sorption on kaolin by the presence of humic acids. Chemosphere 48:1081–1088
Brady NC (1990) The nature and properties of soils, 10th edn. McMillan Pulishing Company, New York, pp 286–291
Chang F, Qu J, Liu H, Liu R, Zhao X (2009) Fe–Mn binary oxide incorporated into diatomite as an adsorbent for arsenite removal: preparation and evaluation. J Colloid Interf Sci 338:353–358
Chen ZS, Lee GJ, Liu JC (2000) The effects of chemical remediation treatments on the extractability and speciation of cadmium and lead in contaminated soils. Chemosphere 41:235–242
Datsyuk V, Kalyva M, Papagelis K, Parthenios J, Tasis D, Siokou A, Kallitsis I, Galiotis C (2008) Chemical oxidation of multiwalled carbon nanotubes. Carbon 46:833–840
de Livera J, McLaughlin MJ, Hettiarachchi GM, Kirby JK, Beak DG (2011) Cadmium solubility in paddy soils: effects of soil oxidation, metal sulfides and competitive ions. Sci Total Environ 409(8):1489–1497
Harvey OR, Herbert BE, Rhue RD, Kuo LJ (2011) Metal interactions at the biocharwaterinterface: energetics and structure-sorption relationships elucidated byflow adsorption microcalorimetry. Environ Sci Technol 45:5550–5556
Hassan AF, Abdel-Mohsen AM, Elhadidy H (2014) Adsorption of arsenic by activated carbon, calcium alginate and their composite beads. Int J Biol Macromol 68:125–130
Heidmann I (2004) Influence of fulvic acid on ion binding and colloidal stability of kaolinite particles. PhD thesis (Diss. ETH no. 15531), Swiss Fed. Inst
Hizal J, Apak R (2006) Modeling of cadmium(II) adsorption on kaolinite-based clays in the absence and presence of humic acid. Appl Clay Sci 32(3–4):232–244
Hu X, Ding Z, Zimmerman AR, Wang S, Gao B (2015) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res 68:206–216
Jiang J, Xu RK, Jiang TY, Li Z (2012) Immobilization of cu (II), Pb (II) and Cd (II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J Hazard Mater 229:145–150
Khan MA, Khan S, Khan A, Alam M (2017) Soil contamination with cadmium, consequences and remediation using organic amendments. Sci Total Environ 601-602:1591–1605
Khan MA, Khan S, Ding X, Khan A, Alam M (2018) The effects of biochar and rice husk on adsorption and desorption of cadmium on to soils with different water conditions (upland and saturated). Chemosphere 193:1120–1126
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides—a review. Environ Pollut 172:9–22
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Manag 28(1):215–225
Li P, Jiang EY, Bai HL (2011) Fabrication of ultrathin epitaxial γ-Fe2O3 films by reactive sputtering. J Phys D Appl Phys 44:075003
Li P, Lang M, Wang XX, Zhang TL (2016) Sorption and desorption of copper and cadmium in a contaminated soil affected by soil amendments. CLEAN Soil Air Water 44(11):1547–1556
Lin LN, Zhou SW, Huang Qg, Huang YC, Qiu WW, Song ZG (2018) Capacity and mechanism of arsenic adsorption on red soil supplemented with ferromanganese oxide–biochar composites. Environ Sci Pollut Res Int 25:20116–20124
Liu WJ, Jiang H, Tian K, Ding YW, Yu HQ (2013) Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ Sci Technol 47:9397–9403
Mohan D, Kumar H, Sarswat A, Alexandre-Franco M, Pittman CU (2014) Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem Eng J 236:513–558
Nesbitt HW, Canning GW, Bancroft GM (1998) XPS study of reduction dissolution of 7A-birnessite by H3AsO3, with constraints on reaction mechanism. Geochim Cosmochim Acta 62:2097–2110
Pradhan M, Bhargava P (2008) Defect and microstructural evolution during drying of soap nut-based alumina foams. J Eur Ceram Soc 28:3049–3057
Rizwan M, Ali S, Adrees M, Rizvi H, Zia-ur-Rehman M, Hannan F, Ok YS (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23(18):17859–17879
Song Z, Lian F, Yu Z, Zhu L, Xing B, Qiu W (2014) Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem Eng J 242:36–42
Tan X, Liu Y, Gu Y, Zeng G, Wang X, Hu X, Sun Z, Yang Z (2015) Immobilization f Cd (II) in acid soil amended with different biochars with a long term of incubation. Environ Sci Pollut Res 22(16):12597–12604
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 20(1):358–368
Xu DY, Zhao Y, Sun K, Gao B, Wang ZY, Jin J, Zhang ZY, Wang SF, Yan Y, Liu XT, Wu FC (2014) Cadmium adsorption on plant- and manure-derived biochar and biochar-amended sandy soils: impact of bulk and surface properties. Chemosphere 111:320–326
Xu Y, Fang Z, Tsang EP (2016) In situ immobilization of cadmium in soil by stabilized biochar-supported iron phosphate nanoparticles. Environ Sci Pollut Res 23(19):19164–19172
Yu Z, Zhou L, Huang Y, Song Z, Qiu W (2015) Effects of a manganese oxide-modified biochar composite on adsorption of arsenic in red soil. J Environ Manag 163:155–162
Zhang S, Niu H, Cai Y, Zhao X, Shi Y (2010) Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4. Chem Eng J 158(3):599–607
Zhou QW, Liao BH, Lin LN, Qiu WW, Song ZG (2018) Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide–biochar composites. Sci Total Environ 615:115–122
Funding
The authors were financially supported by the National Science Foundation of China (41771525) and Central Public Research Institutes Basic Funds for Research and Development (Grant No. Y2017JC10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Zhou, Q., Liao, B., Lin, L. et al. Characteristic of adsorption cadmium of red soil amended with a ferromanganese oxide-biochar composite. Environ Sci Pollut Res 26, 5155–5163 (2019). https://doi.org/10.1007/s11356-018-3942-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3942-6