Abstract
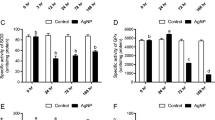
Nanotechnology is a novel arena with promising applications in the field of medicine, industry, and agriculture including fisheries. Cross-disciplinary interactions and the application of this technology in biological systems have led to the innovation of novel nanoparticle antioxidants, which are the subject of our study. In context with above background, we designed an experiment on nano-silver to elucidate its role for mitigation of abiotic and biotic stress. Three diets were formulated viz. silver nanoparticles (Ag-NPs) incorporated at 0.5 and 1 mg/kg diet and control diet (Ag-NPs at 0 mg/kg). Fish were exposed to sublethal level of 1/25th of LC50 (4 ppm) of lead (Pb) and temperature at 34 °C. The effect of Ag-NPs on productive performance (weight gain %, feed conversion ratio, protein efficiency ratio, and specific growth rate), stress biomarkers (catalase, super oxide dismutase, glutathione-s-transferase, acetylcholine esterase, cortisol, heat shock protein), biochemical and immunological response (protein and carbohydrate metabolic enzymes, phagocytic activity, serum total protein and albumin: globulin ratio), histopathology alterations in the liver and gill as well as survival of Channa striatus, following challenge with pathogenic bacteria were evaluated. Dietary Ag-NPs at 0.5-mg supplementation improved growth performance, immunity, survival, and reduced stress biomarker such as HSP 70, cortisol, and blood glucose in various fish tissues. Exposure to Pb and high temperature and group fed with Ag-NPs (1 mg/kg diet) demonstrated remarkable changes in the histo-architect of liver such as pyknotic nuclei, pyknosis, leucocyte infiltration, hemorrhage and karyokinesis, blood vessels with nucleated, lipid vacuoles in the liver tissue. Histology of gill displayed hyperplasia, aneurism, blood congestion, severe telengiectiasis, epithelial lifting, curling of secondary lamella, hyperplasia of epithelial cell of secondary lamella in the group exposed to lead and high temperature and supplemented with Ag-NPs at 1 mg/kg diet. In addition to histopathology, feeding with Ag-NPs at 1 mg/kg diet deteriorated and altered all studied parameters including reduced growth performance. Results obtained in the present study suggest that supplementation of Ag-NPs at 0.5 mg/kg diet has a definitive role to play in the mitigation of abiotic and biotic stress in C. striatus.






Similar content being viewed by others
References
Afifi M, Saddick S, Abu Zinada OA (2016) Toxicity of silver nanoparticles on the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 23(6):754–760
Aklakur M, Rather AM, Kumar N (2016) Nano delivery: an emerging avenue for Nutraceuticals and drug delivery. Crit Rev Food Sci Nutr 56(14):2352–2361. https://doi.org/10.1080/10408398.2013.839543
Ali A, Bharadwaj S, O'Carroll R, Ovsenek N (1998) HSP90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol Cell Biol 18:4949–4960
Almofti MR, Ichikawa T, Yamashita K, Terada H, Shinohara Y (2003) Silver ion induces a cyclosporine a-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome C. J Biochem 134:43–49
APHA-AWWA-WEF (1998) In: Clesceri LS, Greenberg AE, Eaton AD (eds) Standard methods for the estimation of water and waste water, 20th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC
Beck I, Hotowy A, Sawosz E, Grodzik M, Wierzbicki M, Kutwin M, Jaworski S, Chwalibog A (2015) Effect of silver nanoparticles and hydroxyproline, administered in ovo, on the development of blood vessels and cartilage collagen structure in chicken embryos. Arch Anim Nutr 69:57–68
Brio PA (1998) Staying cool: behavioral thermoregulation during summer by young-of-year brook trout in a lake. Trans Am Fish Soc 127:212–222
Chakraborty C, Pal S, Doss GP, Wen ZH, Lin C (2013) Nanoparticles as ‘smart’ pharmaceutical delivery. Front Biosci 18:1030–1050
Doumas BT, Watson W, Biggs HG (1971) Albumin standards and measurement of serum albumin with bromocresol green. Clin Chim Acta 31:87–96
Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ (2005) Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol 3:6
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ (eds) Fish physiology: environmental relations and behavior. Academic Press, New York, pp 1–98
Giulio RT, Di Washburn PC, Wenning RJ, Winston GW, Jewell CS (1989) Biochemical responses in aquatic animals: a review of oxidative stress. Environ Toxicol Chem 8:1103–1123
Gong Z, Yang J, Yang M, Wang F, Wei Q, Tanguay RM, Wu T (2006) Benzo (a) pyrene inhibits expression of inducible heat shock protein 70 in vascular endothelial cells. Toxicol Lett 166:229–236
Govindasamy R, Rahuman AA (2012) Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J Environ Sci (China) 24(6):1091–1098
Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86:377–384
Gupta SK, Pal AK, Sahu NP, Saharan N, Chandraprakash AMS, Kumar S (2014) Haemato-biochemical responses in Cyprinus carpio(Linnaeus, 1758) fry exposed to sub-lethal concentration of a phenylpyrazole insecticide, fipronil. Proc Natl Acad Sci India Sect B Biol Sci 84(1):113–122
Haase H, Fahmi A, Mahltig BJ (2014) Impact of silver nanoparticles and silver ions on innate immune cells. J Biomed Nanotechnol 10(6):1146–1156
Habing WH, Pabst MN, Bjacoby W, Glutathion S (1974) Glutathione S-transferases. Transferase, the first enzymatic step in mercatpopunc acid formation. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge JMC (1999) In: free radicals in biology and medicine. Oxford University Press, Oxford, p 543
Hestrin S (1949) The reaction of acetyl choline and other carboxylic acid derivatives with hydroxylamine and its analytical application. J Biol Chem 180:249–261
Hong T, Tripathy N, Son H, Ha K, Jeong H, Hahn YA (2013) Comprehensive in vitro and in vivo study of ZnO nanoparticles toxicity. J Mater Chem B 1(23):2985–2992
Johari SA, Kalbassi MR, Soltani M, Yu IJ (2013) Toxicity comparison of colloidal silver nanoparticles in various life stages of rainbow trout (Oncorhynchus mykiss). Iran J Fish Sci 12:76–95
Khan MS, Qureshi NA, Jabeen F (2017) Assessment of toxicity in fresh water fish Labeo rohita treated with silver nanoparticles. Appl Nanosci 7:167–179. https://doi.org/10.1007/s13204-017-0559-x
Kock G, Triendl M, Hofer R (1996) Seasonal patterns of metal accumulation in Arctic char (Salvelinus alpinus) from an oligotrophic alpine lake related to temperature. Can J Fish Aqua Sci 53:780–786
Kumar N, Gupta S, Chandan NK, Aklakur M, Pal AK, Jadhao SB (2014) Lipotropes protect against pathogen-aggravated stress and mortality in low dose pesticide-exposed fish. PLoS One 9(4):e93499
Kumar N, Ambasankar K, Krishnani KK, Bhushan S, Minhas PS (2016a) Dietary pyridoxine protects against stress and maintains immune-hematological status in Chanos chanos exposed to endosulfan. Basic Clin Pharmacol Toxicol 119(3):297–308
Kumar N, Ambasankar K, Krishnani KK, Gupta SK, Bhushan S, Minhas PS (2016b) Acute toxicity, biochemical and histopathological responses of endosulfan in Chanos chanos. Ecotoxicol Environ Saf 131:79–88
Kumar N, Krishnani KK, Brahmane MP, Gupta SK, Kumar P, Singh NP (2017a) Temperature induces lead toxicity in Pangasius hypophthalmus: an acute test, antioxidative status and cellular metabolic stress. Int J Environ Sci Technol 15:57–68. https://doi.org/10.1007/s13762-017-1364-5
Kumar N, Krishnani KK, Kumar P, Singh NP (2017b) Zinc nanoparticles potentiates thermal tolerance and cellular stress protection of Pangasius hypophthalmus reared under multiple stressors. J Therm Biol 70:61–68
Kumar N, Krishnani KK, Gupta SK, Singh NP (2017c) Selenium nanoparticles enhanced thermal tolerance and maintain cellular stress protection of Pangasius hypophthalmus reared under lead and high temperature. Respir Physiol Neurobiol 246:107–116
Kumar N, Krishnani KK, Kumar P, Jha AK, Gupta SK, Singh NP (2017d) Dietary zinc promotes immuno-biochemical plasticity and protects fish against multiple stresses. Fish Shellfish Immunol 62:184–194
Kumar N, Krishnani KK, Singh NP (2018) Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes and histopathological response in fish. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-1165-x
Lansdown AB (2006) Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol 33:17–34
Lowry OH, Ronebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–276
Martinez-Gutierrez F, Thi EP, Silverman JM, de Oliveira CC, Svensson SL, AHoek V, Sanchez EM, Reiner NE, Gaynor EC, Pryzdial EL, Conway EM, Orrantia E, Ruiz F, Av-Gay Y, Bach H (2012) Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomedicine 8(3):328–336
Martinez-Porchas M, Martinez-Cordova LR, Ramos-Enriquez R (2009) Cortisol and glucose: reliable indicators of fish stress? Panam. J Aquat Sci 4(2):158–178
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Morimoto RI, Tissieres A, Cesrgopoulos C (1993) Cell in stress: transcriptional activation of heat shock genes. Science 259:1409–1410
Moyle PB, Cech JJ (2004) Fishes: an introduction to ichthyology, 5th edn. Prentice Hall, Englewood Cliffs
Murty AS (1986) Toxicity of pesticides to fish. Vols. I and II. Boca Raton, CRC Press, pp 355–483
Muthappa NA, Gupta S, Yengkokpam S, Debnath D, Kumar N, Pal AK, Jadhao SB (2014) Lipotropes promote immunobiochemical plasticity and protect fish against low-dose pesticide-induced oxidative stress. Cell Stress Chaperon 19(1): 61–81.
Myrzakhanova M, Gambardella C, Falugi C, Gatti AG, Tagliafierro G, Ramoino P, Bianchini P, Diaspro A (2013) Effects of nanosilver exposure on cholinesterase activities, CD41, and CDF/LIF-like expression in ZebraFish (Danio rerio) larvae. Biomed Res Int. https://doi.org/10.1155/2013/205183
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Nemcsok J, Orban L, Asztalos B, Vig E (1987) Accumulation of pesticides in the organs of carp, Cyprinus carpio L., at 4 and 20 °C. Bull Environ Contam Toxicol 39(3):370–378
Ochoa S (1955) Malic dehydrogenase and ‘Malic’ enzyme. In: Coloric SP, Kaplan N (eds) Methods of enzymology, Vol I. Academic Press, New York, pp 735–745
Percival SL, Bowler PG, Dolman J (2007) Antimicrobial activity of silver containing dressings on wound microorganisms using an in vitro biofilm model. Int Wound J 4(2):186–191
Rajkumar KS, Kanipandian N, Thirumurugan R (2016) Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci 6(1):19–29
Roberts RJ (1989) Nutritional pathology of teleosts. In: Roberts RJ (ed) Fish pathology. Bailliere Tindall, London, pp 337–362
Samuel U, Guggenbichler JP (2004) Prevention of catheter-rel the potential of a new nano silver impregnated catheter. Int J Antimicrob Agents 23S1:S75–S78
Sarkar B, Kumar M, Verma S, Rathore RM (2015) Effect of dietary nanosilver on gut proteases and general performance in Zebrafish (Danio rerio). Int J Aqut Biol 3(2):60–67
Secombes CJ (1990) Isolation of salmonid macrophage and analysis of their killing activity. In: Stolen JSTC, Fletcher DP, Anderson BS, Van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publication, Fair Haven, pp 137–152
Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S (2007) Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 3(2):168–171
Shaluei F, Hedayati A, Jahanbakhshi A, Kolangi H, Fotovat M (2013) Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix). Hum Exp Toxicol 32(12):1270–1277. https://doi.org/10.1177/0960327113485258
Sinha T, Ahmaruzzaman M (2015) A novel and greener approach for shape controlled synthesis of gold and gold–silver core shell nanostructure and their application in optical coatings. Spectrochimica Acta Part A: Mol Biomol Spectrosc 145:280–288. https://doi.org/10.1016/j.saa.2015.03.059
Sinha T, Ahmaruzzaman M, Sil AK, Bhattacharjee A (2014) Biomimetic synthesis of silver nanoparticles using the fish scales of Labeo rohita and their application as catalysts for the reduction of aromatic nitro compounds. Spectrochim Acta A Mol Biomol Spectrosc 131:413–423. https://doi.org/10.1016/j.saa.2014.04.065
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Somoyogi M (1945) A new reagent for the determination of sugars. J Biol Chem 160:61–68
Stasiack AS, Bauman CP (1996) Neutrophil activity as a potent indicator concomitant analysis. Fish Shellfish Immunol 37:539
Takahara S, Hamilton BH, Nell JV, Kobra TY, Ogura Y, Nishimura ET (1960) Hypocatalesemia, a new generis carrier state. J Clin Investig 29:610–619
Wiegertjes GF, Stet RJM, Parmentier HK, Van WB (1996) Muiswinkel, Immunogenetics of disease resistance in fish; a comparable approach. Dev Comp Immunol 20:365–381
Wootton IDP (1964) Microanalysis in medical biochemistry. J & A Churchill Ltd., London, pp 101–103
Wroblewski L, LaDue JS (1955) Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med 90:210–213
Yang P, Wei W, Tao C (2007) Determination of trace thiocyanate with nano-silver coated multi-walled carbon nanotubes modified glassy carbon electrode. Anal Chim Acta 585:331–336
Acknowledgements
Authors express sincere gratitude to the Director, ICAR-National Institute of abiotic Stress Management, Baramati, Pune for providing all the facilities to conduct the present work. The financial assistance has been provided by Indian Council of Agricultural Research (ICAR), New Delhi, India as institutional project (#IXX09651) is highly acknowledged. I also place a record of thanks to Mrs. Yogita and Mr. Yuvraj Sanas for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kumar, N., Krishnani, K.K., Gupta, S.K. et al. Effects of silver nanoparticles on stress biomarkers of Channa striatus: immuno-protective or toxic?. Environ Sci Pollut Res 25, 14813–14826 (2018). https://doi.org/10.1007/s11356-018-1628-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1628-8