Abstract
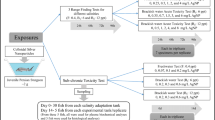
Silver nanoparticles (AgNPs) are increasingly used in several industrial and household products because of their antibacterial and antifungal properties. Hence, there is an inevitable risk that these chemicals may end up in aquatic biotopes and have adverse effects on the fauna. In order to assess potential health effects on aquatic organisms, this study evaluated the effects of waterborne AgNP exposure for 7 days on a set of critical stress parameters in juvenile Caspian kutum (Rutilus kutum), an economically important fish in the Caspian Sea. The applied level 11 μg/l of AgNP is high compared to reported water concentrations and corresponds to 40% of the 96 h LC50 value, initially determined to be 28 μg/l. Gill heat shock protein 70 (hsp70) mRNA expression, Na+/K+-ATPase activity and enzymatic activities of liver superoxide dismutase (SOD), glutathione peroxidase (Gpx), lactate dehyrogenase (LDH) and alkaline phosphatase (ALP), and whole-body cortisol and thyroid hormones (T3 and T4) were measured as endpoints. Gill hsp70 mRNA expression increased and gill Na+/K+-ATPase activity decreased in AgNP-exposed fish compared to controls. The specific activities of all liver enzymes decreased significantly compared to controls. Whole-body cortisol and thyroid hormones decreased compared to controls. In conclusion, the study demonstrates that AgNPs cause oxidative stress and gill osmoregulatory disruption in Caspian kutum juveniles.


Similar content being viewed by others
References
Ahamed, M., Posgai, R., Gorey, T. J., Nielsen, M., Hussain, S. M., & Rowe, J. J. (2010). Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicology and Applied Pharmacology, 242(3), 263–269.
Almeida, J., Novelli, E., Silva, M. D. P., & Júnior, R. A. (2001). Environmental cadmium exposure and metabolic responses of the Nile tilapia, Oreochromis niloticus. Environmental Pollution, 114(2), 169–175.
Aluru, N., Jorgensen, E. H., Maule, A. G., & Vijayan, M. M. (2004). PCB disruption of the hypothalamus-pituitary-interrenal axis involves brain glucocorticoid receptor downregulation in anadromous Arctic charr. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 287(4), R787–R793.
Antognelli, C., Romani, R., Baldracchini, F., De Santis, A., Andreani, G., & Talesa, V. (2003). Different activity of glyoxalase system enzymes in specimens of Sparus auratus exposed to sublethal copper concentrations. Chemico-Biological Interactions, 142(3), 297–305.
Aydın, R., & Köprücü, K. (2005). Acute toxicity of diazinon on the common carp (Cyprinus carpio L.) embryos and larvae. Pesticide Biochemistry and Physiology, 82(3), 220–225.
Bacchetta, C., Ale, A., Simoniello, M. F., Gervasio, S., Davico, C., Rossi, A. S., Desimone, M. F., Poletta, G., López, G., Monserrat, J. M., & Cazenave, J. (2017). Genotoxicity and oxidative stress in fish after a short-term exposure to silver nanoparticles. Ecological Indicators, 76, 230–239.
Banaee, M., Sureda, A., Zohiery, F., Hagi, B. N., & Garanzini, D. S. (2014). Alterations in biochemical parameters of the freshwater fish, Alburnus mossulensis, exposed to sublethal concentrations of fenpropathrin. International Journal of Aquatic Biology, 2(2), 58–68.
Banaee, M., Sureda, A., Shahaf, S., & Fazilat, N. (2015). Protective effects of silymarin extract on malthion-induced zebra cichlid (Cichlasoma nigrofasciatum) hepatotoxicity. Iranian Journal of Toxicology, 9(28), 1239–1246.
Barnes, J., Zheng, Y., & Lyons, T. (2002). Plant resistance to ozone: the role of ascorbate. In K. Omasa, H. Saji, S. Youssefian, & N. Kondo (Eds.), Air pollution and plant biotechnology (pp. 235–252). New York: Springer.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44(1), 276–287.
Beer, C., Foldbjerg, R., Hayashi, Y., Sutherland, D. S., & Autrup, H. (2012). Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicology Letters, 208, 286–292.
Bianchini, A., Grosell, M., Gregory, S. M., & Wood, C. M. (2002). Acute silver toxicity in aquatic animals is a function of sodium uptake rate. Environmental Science & Technology, 36(8), 1763–1766.
Bilberg, K., Hovgaard, M. B., Besenbacher, F., & Baatrup, E. (2012). In vivo toxicity of silver nanoparticles and silver ions in zebrafish (Danio rerio). Journal of Toxicology. doi:10.1155/2012/293784.
Carew, A. C., Hoque, M. E., Metcalfe, C. D., Peyrot, C., Wilkinson, K. J., & Helbing, C. C. (2015). Chronic sublethal exposure to silver nanoparticles disrupts thyroid hormone signaling during Xenopus laevis metamorphosis. Aquatic Toxicology, 159, 99–108.
Chae, Y. J., Pham, C. H., Lee, J., Bae, E., Yi, J., & Gu, M. B. (2009). Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes). Aquatic Toxicology, 94(4), 320–327.
Choi, J. E., Kim, S., Ahn, J. H., Youn, P., Kang, J. S., Park, K., Yi, J., & Ryu, D. Y. (2010). Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquatic Toxicology, 100(2), 151–159.
Farmen, E., Mikkelsen, H., Evensen, Ø., Einset, J., Heier, L., Rosseland, B., Salbu, B., Tollefsen, K. E., & Oughton, D. H. (2012). Acute and sub-lethal effects in juvenile Atlantic salmon exposed to low μg/l concentrations of Ag nanoparticles. Aquatic Toxicology, 108, 78–84.
Ghaninejad, D., Moghim, M., Burani, S., & Abdolmaleki, S. (2001). Stock assessment of bony fish in the Caspian Sea. Final report. Bony Fish Research Center. p. 98.
Girilal, M., Krishnakumar, V., Poornima, P., Fayaz, A. M., & Kalaichelvan, P. (2015). A comparative study on biologically and chemically synthesized silver nanoparticles induced heat shock proteins on fresh water fish Oreochromis niloticus. Chemosphere, 139, 461–468.
Gottschalk, F., Ort, C., Scholz, R. W., & Nowack, B. (2011). Engineered nanomaterials in rivers—exposure scenarios for Switzerland at high spatial and temporal resolution. Environmental Pollution, 159, 3439–3445.
Griffitt, R. J., Hyndman, K., Denslow, N. D., & Barber, D. S. (2009). Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicological Sciences, 107(2), 404–415.
Grosell, M., Brauner, C. J., Kelly, S. P., McGeer, J. C., Bianchini, A., & Wood, C. M. (2002). Physiological responses to acute silver exposure in the freshwater crayfish (Cambarus diogenes diogenes)—a model invertebrate? Environmental Toxicology and Chemistry, 21(2), 369–374.
Hartl, F. U., & Hayer-Hartl, M. (2002). Molecular chaperones in the cytosol: from nascent chain to folded protein. Science, 295(5561), 1852–1858.
Hontela, A. (2006). Corticosteroidogenesis and StAR protein of rainbow trout disrupted by human-use pharmaceuticals: data for use in risk assessment. Toxicological Sciences, 93(1), 1–2.
Iwama, G. K., Thomas, P. T., Forsyth, R. B., & Vijayan, M. M. (1998). Heat shock protein expression in fish. Reviews in Fish Biology and Fisheries, 8(1), 35–56.
Jang, M. H., Kim, W. K., Lee, S. K., Henry, T. B., & Park, J. W. (2014). Uptake, tissue distribution, and depuration of total silver in common carp (Cyprinus carpio) after aqueous exposure to silver nanoparticles. Environmental Science & Technology, 48(19), 11568–11574.
Joo, H. S., Kalbassi, M. R., Yu, I. J., Lee, J. H., & Johari, S. A. (2013). Bioaccumulation of silver nanoparticles in rainbow trout (Oncorhynchus mykiss): influence of concentration and salinity. Aquatic Toxicology, 140, 398–406.
Katsumiti, A., Gilliland, D., Arostegui, I., & Cajaraville, M. P. (2015). Mechanisms of toxicity of Ag nanoparticles in comparison to bulk and ionic Ag on mussel hemocytes and gill cells. PloS One, 10(6), e0129039.
Katuli, K. K., Massarsky, A., Hadadi, A., & Pourmehran, Z. (2014). Silver nanoparticles inhibit the gill Na+/K+-ATPase and erythrocyte AChE activities and induce the stress response in adult zebrafish (Danio rerio). Ecotoxicology and Environmental Safety, 106, 173–180.
Kim, S., & Ryu, D. Y. (2013). Silver nanoparticle induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. Journal of Applied Toxicology, 33(2), 78–89.
Kime, D. (1978). The hepatic catabolism of cortisol in teleost fish—adrenal origin of 11-oxotestosterone precursors. General and Comparative Endocrinology, 35(3), 322–328.
Kroglund, F., Rosseland, B., Teien, H.-C., Salbu, B., Kristensen, T., & Finstad, B. (2007). Water quality limits for Atlantic salmon (Salmo salar L.) exposed to short term reductions in pH and increased aluminum simulating episodes. Hydrology and Earth System Sciences, 12, 491–507.
Lee, K. J., & Hahn, G. M. (1988). Abnormal proteins as the trigger for the induction of stress responses: heat, diamide, and sodium arsenite. Journal of Cellular Physiology, 136(3), 411–420.
Lee, B., Duong, C. N., Cho, J., Lee, J., Kim, K., Seo, Y., Kim, P., Choi, K., & Yoon, J. (2012). Toxicity of citrate-capped silver nanoparticles in common carp (Cyprinus carpio). Journal of Biomedicine and Biotechnology. doi:10.1155/2012/262670.
Li, H., Zhang, J., Wang, T., Luo, W., Zhou, Q., & Jiang, G. (2008). Elemental selenium particles at nano-size (nano-Se) are more toxic to medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: a comparison with sodium selenite. Aquatic Toxicology, 89(4), 251–256.
Li, H., Zhou, Q., Wu, Y., Fu, J., Wang, T., & Jiang, G. (2009). Effects of waterborne nano-iron on medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicology and Environmental Safety, 72(3), 684–692.
Li, Z.-H., Chen, L., Wu, Y.-H., Li, P., Li, Y.-F., & Ni, Z.-H. (2014). Effects of waterborne cadmium on thyroid hormone levels and related gene expression in Chinese rare minnow larvae. Comparative biochemistry and physiology Part C. Toxicology & Pharmacology, 161, 53–57.
Lopez, C., Pineiro, A., Nunez, N., Avagnina, A., Villaamil, E., & Roses, O. (2000). Thyroid hormone changes in males exposed to lead in the Buenos Aires area (Argentina). Pharmacological Research, 42(6), 599–602.
Lower, N., Moore, A., Scott, A., Ellis, T., James, J., & Russell, I. (2005). A non-invasive method to assess the impact of electronic tag insertion on stress levels in fishes. Journal of Fish Biology, 67(5), 1202–1212.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193(1), 265–275.
Martínez-Porchas, M., Martínez-Córdova, L. R., & Ramos-Enriquez, R. (2009). Cortisol and glucose: reliable indicators of fish stress. Pan-American Journal of Aquatic Sciences, 4(2), 158–178.
Maynard, A. D., Aitken, R. J., Butz, T., Colvin, V., Donaldson, K., Oberdörster, G., Philbert, M. A., Ryan, J., Seaton, A., Stone, V., Tinkle, S. S., Tran, L., Walker, N. J., & Warheit, D. B. (2006). Safe handling of nanotechnology. Nature, 444(7117), 267–269.
McCormick, S. D. (1993). Methods for nonlethal gill biopsy and measurement of Na+,K+-ATPase activity. Canadian Journal of Fisheries and Aquatic Sciences, 50, 656–658.
Miao, W., Zhu, B., Xiao, X., Li, Y., Dirbaba, N. B., Zhou, B., & Wu, H. (2015). Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquatic Toxicology, 161, 117–126.
Musee, N., Thwala, M., & Nota, N. (2011). The antibacterial effects of engineered nanomaterials: implications for wastewater treatment plants. Journal of Environmental Monitoring, 13, 1164–1183.
Nebeker, A. V., McAuliffe, C. K., Mshar, R., & Stevens, D. G. (1983). Toxicity of silver to steelhead and rainbow trout, fathead minnows and Daphnia magna. Environmental Toxicology and Chemistry, 2(1), 95–104.
OECD. (2000). Guideline for the testing of chemicals No. 215. Fish, juvenile growth test. Paris: Organisation for Economic Co-operation and Development.
Ostrander, G. K. (2000). The laboratory fish. London: Academic Press.
Peter, M. S. (2011). The role of thyroid hormones in stress response of fish. General and Comparative Endocrinology, 172(2), 198–210.
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RTPCR. Nucleic Acids Research, 29(9), e45–e45.
Pham, C. H., Yi, J., & Gu, M. B. (2012). Biomarker gene response in male Medaka (Oryzias latipes) chronically exposed to silver nanoparticle. Ecotoxicology and Environmental Safety, 78, 239–245.
Rajkumar, K., Kanipandian, N., & Thirumurugan, R. (2016). Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles exposed freshwater fish Labeo rohita. Applied Nanoscience, 6(1), 19–29.
Reidy, B., Haase, A., Luch, A., Dawson, K. A., & Lynch, I. (2013). Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials, 6(6), 2295–2350.
Rothman, J. E. (1989). Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell, 59(4), 591–601.
Schultz, A. G., Ong, K. J., MacCormack, T., Ma, G., Veinot, J. G., & Goss, G. G. (2012). Silver nanoparticles inhibit sodium uptake in juvenile rainbow trout (Oncorhynchus mykiss). Environmental Science & Technology, 46(18), 10295–10301.
Shalulei, F., Hedayati, A., Jahanbakhshi, A., Kolangi, H., & Fotovat, M. (2013). Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix). Human and Experimental Toxicology. doi:10.1177/0960327113485258.
Sharyati, A. (1993). Fish of the Caspian Sea region (pp. 77–79). Iran: Iranian Fisheries Company.
Shrimpton, J. M., & Mccormick, S. D. (1999). Responsiveness of gill Na+/K+-ATPase to cortisol is related to gill corticosteroid receptor concentration in juvenile rainbow trout. Journal of Experimental Biology, 202(8), 987–995.
Siddhuraju, P., & Becker, K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. Journal of Agricultural and Food Chemistry, 51(8), 2144–2155.
Singh, R. K., & Sharma, B. (1998). Carbofuran induced biochemical changes in Clarias batrachus. Pesticide Science, 53(4), 285–290.
Sink, T. D., Kumaran, S., & Lochmann, R. T. (2007). Development of a whole-body cortisol extraction procedure for determination of stress in golden shiners, Notemigonus crysoleucas. Fish Physiology and Biochemistry, 33(3), 189–193.
Teodorescu, D., Munteanu, M. C., Staicu, A. C., & Dinischiotu, A. (2012). Changes in lactate dehydrogenase activity in Carassius auratus gibelio (L. Pysces) kidney, gills and intestine induced by acute exposure to copper. Romanian Biotechnological Letters, 17(6), 7873–7880.
Völker, C., Oetken, M., & Oehlmann, J. (2013). The biological effects and possible modes of action of nanosilver. In D. M. Whitacre (Ed.), Reviews of environmental contamination and toxicology Vol 223 (pp. 81–106). New York: Springer.
Völker, C., Kämpken, I., Boedicker, C., Oehlmann, J., & Oetken, M. (2015). Toxicity of silver nanoparticles and ionic silver: comparison of adverse effects and potential toxicity mechanisms in the freshwater clam Sphaerium corneum. Nanotoxicology, 9(6), 677–685.
Wang, J., & Wang, W.-X. (2014). Low bioavailability of silver nanoparticles presents trophic toxicity to marine medaka (Oryzias melastigma). Environmental Science & Technology, 48(14), 8152–8161.
Wigger, H. (2017). Risk assessment of technological innovations. In Environmental release of and exposure to iron oxide and silver nanoparticles (pp. 11–50). Wiesbaden: Springer Fachmedien. doi:10.1007/978-3-658-16791-2_2.
Wood, C. M., Playle, R. C., & Hogstrand, C. (1999). Physiology and modeling of mechanisms of silver uptake and toxicity in fish. Environmental Toxicology and Chemistry, 18(1), 71–83.
Wu, Y., & Zhou, Q. (2013). Silver nanoparticles cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days of exposure. Environmental Toxicology and Chemistry, 32(1), 165–173.
Yang, J., Chen, G., Huang, J.-s., Zhang, J.-d., Shi, G., Tang, B.-g., & Zhou, H. (2007). Effects of temperature and salinity on the growth and activities of antioxidant enzymes of cobia (Rachycentron canadum) juveniles. Journal of Guangdong Ocean University, 2007-04.
Acknowledgements
The authors acknowledge the Department of Biology of University of Southern Denmark and Department of Biochemistry of University of Guilan, Iran, for providing necessary lab facilities to carry out the work successfully. This research was supported by the University of Tehran (grant # 6/31/2703010). SSM was supported also by a grant from The Danish Research Council for Independent Research (DFF-4181-00020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masouleh, F.F., Amiri, B.M., Mirvaghefi, A. et al. Silver nanoparticles cause osmoregulatory impairment and oxidative stress in Caspian kutum (Rutilus kutum, Kamensky 1901). Environ Monit Assess 189, 448 (2017). https://doi.org/10.1007/s10661-017-6156-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6156-3