Abstract
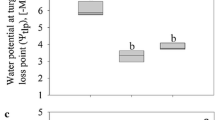
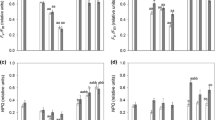
Tropospheric ozone (O3) causes severe damage to many vascular plants but not to lichens. It was recently suggested that this may be due to their high levels of natural defences against the oxidative bursts associated to their fluctuating water content. In this study, the combined effects of watering regime (with or without a daily spray of distilled water), air relative humidity (20 ± 5 vs. 80 ± 5% RH) and O3 (250 vs. 0 ppb, 5 h day−1 for 2 weeks) were monitored in two chlorolichens with different ecology, Parmotrema perlatum and Xanthoria parietina. Modulated chlorophyll a fluorescence (Chl a F), superoxide anion radical (O2 •−) and hydrogen peroxide (H2O2) production, antioxidant content and enzyme activity of the ascorbate/glutathione cycle were measured after exposure and, for Chl a F, after 1 and 2 days of recovery. The species differed in the antioxidant profile (ascorbate was higher in X. parietina, glutathione in P. perlatum), and in the activity of ROS-scavenging enzymes, more intense in the hygrophilous P. perlatum than in the meso-xerophilous X. parietina. O3 slightly modified Chl a F parameters related to the controlled dissipation, with reduction of Fm, Fv/Fm (both species) and ETR (in P. perlatum), and increase in NPQ and qN (in X. parietina). It also influenced, particularly in P. perlatum, the content of H2O2, glutathione (GSH) and oxidized glutathione (GSSG) (but not that of O2 •− and AsA + DHA) and the activity of superoxide dismutase, ascorbate peroxidase and dehydroascorbate reductase. These parameters, however, were more heavily affected by water availability. The hypothesis that lichens are O3-tolerant thanks to the constitutive antioxidant systems, intimately related to their poikilohydric life-style, is thus confirmed.



Similar content being viewed by others
References
Able AJ, Guest DI, Sutherland MW (1998) Use of a new tetrazolium–based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var. nicotianae. Plant Physiol 117:491–499
Ahmadjian V (1973) Methods of isolation and culturing lichen symbionts and thalli. In: Ahmadjian V, Hale ME (eds) The lichens. Academic Press, New York, pp 653–659
Ahmadjian V (1993) The lichen symbiosis. Wiley, Oxford
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Barták M, Trnková K, Hansen ES, Hazdrová J, Skácelová K, Hájek J, Forbelská M (2015) Effect of dehydration on spectral reflectance and photosynthetic efficiency in Umbilicaria arctica and U. hyperborea. Biol Plant 59:357–365
Bertuzzi S, Tretiach M (2013) Hydrogen sulphide inhibits PSII of lichen photobionts. Lichenologist 45:101–113
Bertuzzi S, Davies L, Power SA, Tretiach M (2013) Why lichens are bad biomonitors of ozone pollution? Ecol Indic 34:391–397
Betzelberger AM, Yendrek CR, Sun J, Leisner CP, Nelson RL, Ort DR, Ainsworth EA (2012) Ozone exposure response for U.S. soybean cultivars: linear reductions in photosynthetic potential, biomass, and yield. Plant Physiol 160:1827–1839
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Bussotti F, Desotgiu R, Cascio C, Pollastrini M, Gravano E, Gerosa G, Marzuoli R, Nali C, Lorenzini G, Salvatori E, Manes F, Schaub M, Strasser RJ (2011) Ozone stress in woody plants assessed with chlorophyll a fluorescence. A critical reassessment of existing data. Environ Exp Bot 73:19–30
Calatayud A, Deltoro VI, Barreno E, del Valle-Tascon S (1997) Changes in in vivo chlorophyll fluorescence quenching in lichen thalli as a function of water content and suggestion of zeaxanthin-associated photoprotection. Physiol Plant 101:93–102
Calatayud A, Temple PJ, Barreno E (2000) Modulated chlorophyll a fluorescence emission, xanthophyll cycle activity, and net photosynthetic rate responses to ozone in some foliose and fruticose lichen species. Photosynthetica 38:281–286
Candotto Carniel F, Zanelli D, Bertuzzi S, Tretiach M (2015) Desiccation tolerance and lichenization: a case study with the aeroterrestrial microalga Trebouxia sp. (Chlorophyta). Planta 242:493–505
Candotto Carniel F, Gerdol M, Montagner A, Banchi E, De Moro G, Manfrin C, Muggia L, Pallavicini A, Tretiach M (2016) New features of desiccation tolerance in the lichen photobiont Trebouxia gelatinosa are revealed by a transcriptomic approach. Plant Mol Biol 91:319–339
Carrasco-Rodriguez JL, del Valle-Tascon S (2001) Impact of elevated ozone on chlorophyll a fluorescence in field-grown oat (Avena sativa). Environ Exp Bot 45:133–142
Ciompi S, Castagna A, Ranieri A, Nali C, Lorenzini G, Soldatini GF (1997) CO2 assimilation, xanthophyll cycle pigments and PSII efficiency in pumpkin plants as affected by ozone fumigation. Physiol Plant 101:881–889
Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27:959–970
Cruz de Carvalho R, Catalá M, da Silva JM, Branquinho C, Barreno E (2012) The impact of dehydration rate on the production and cellular location of reactive oxygen species in an aquatic moss. Ann Bot 110:1007–1016
del Hoyo A, Álvarez R, del Campo EM, Gasulla F, Barreno E, Casano LM (2011) Oxidative stress induces distinct physiological responses in the two Trebouxia phycobionts of the lichen Ramalina farinacea. Ann Bot 107:109–118
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228
Döring AS, Pellegrini E, Campanella A, Trivellini A, Gennai C, Petersen M, Nali C, Lorenzini G (2014) How sensitive is Melissa officinalis to realistic ozone concentrations? Plant Physiol Biochem 74:156–164
Feng Z, Paoletti E, Bytnerowicz A, Harmens H (2015) Ozone and plants. Environ Pollut. doi:10.1016/j.envpol.2015.02.004
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Fuhrer J (2009) Ozone risk for crops and pastures in present and future climates. Naturwissenschaften 96:173–194
Gillham DJ, Dodge AD (1986) Hydrogen–peroxide–scavenging system within pea chloroplasts. Planta 167:246–251
Gottardini E, Cristofori A, Cristofolini F, Nali C, Pellegrini E, Bussotti F, Ferretti M (2014) Chlorophyll–related indicators are linked to visible ozone symptoms: evidence from a field study on native Viburnum lantana L. plants in northern Italy. Ecol Indic 39:65–74
Gries C, Sanz MJ, Nash TH III (1995) The effect of SO2 fumigation on CO2 gas exchange, chlorophyll fluorescence and chlorophyll degradation in different lichen species from western North America. Crypt Bot 5:239–246
Heber U, Bilger W, Bligny R, Lange OL (2000) Phototolerance of lichens, mosses and higher plants in an alpine environment: analysis of photoreactions. Planta 211:770–780
Jensen M (2002) Measurement of chlorophyll fluorescence in lichens. In: Kranner I, Beckett RP, Varma AK (eds) Protocols in lichenology. Culturing, biochemistry, ecophysiology and use in biomonitoring. Springer, Berlin, Heidelberg, pp 135–151
Jonsson AV, Moen J, Palmqvist K (2008) Predicting lichen hydration using biophysical models. Oecologia 156:259–273
Kawakami S, Mizuno M, Tsuchida H (2000) Comparison of antioxidant enzyme activities between Solanum tuberosum L. cultivars Danshaku and Kitaakari during low–temperature storage. J Agric Food Chem 48:2117–2121
Kosugi M, Arita M, Shizuma R, Moriyama Y, Kashino Y, Koike H, Satoh K (2009) Responses to desiccation stress in lichens are different from those in their photobionts. Plant Cell Physiol 50:879–888
Kosugi M, Miyake A, Kasino Y, Shibata Y, Satoh K, Itoh S (2013) Lichens assist the drought–induced fluorescence quenching of their photobiont green algae through arabitol. In: Kuang T, Lu C, Zhang L (eds) Photosynthesis research for food, fuel and the future. Springer, Berlin–Heidelberg, pp 514–517
Kranner I (2002) Glutathione status correlates with different degrees of desiccation tolerance in three lichens. New Phytol 154:451–460
Kranner I, Grill D (1996) Significance of thiol–disulfide exchange in resting stages of plant development. Bot Acta 109:8–14
Kranner I, Cram WJ, Zorn M, Wornik S, Yoshimura I, Stabentheiner E, Pfeifhofer HW (2005) Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. PNAS 102:3141–3146
Kranner I, Birtic S, Anderson KM, Pritchard HW (2006) Glutathione halfcell reduction potential: a universal stress marker and modulator of programmed cell death? Free Radic Biol Med 40:2155–2165
Kranner I, Beckett R, Hochman A, Nash TH III (2008) Desiccation–tolerance in lichens: a review. Bryologist 111:576–593
Krause GH, Jahns P (2004) Non–photochemical energy dissipation determined by chlorophyll fluorescence quenching: characterization and function. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Berlin–Heidelberg, pp 463–495
Laisk A, Kull O, Moldau H (1989) Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol 90:1163–1167
Lange OL, Green TG (2008) Diel and seasonal courses of ambient carbon dioxide concentration and their effect on productivity of the epilithic lichen Lecanora muralis in a temperate, suburban habitat. Lichenologist 40:449–462
Lange OL, Kilian E (1985) Reaktivierung der Photosynthese trockener Flechten durch Wasserdampfaufnahme aus dem Luftraum: Artspezifisch unterschiedliches Verhalten. Flora 176:7–23
Lange OL, Kilian E, Ziegler H (1986) Water vapor uptake and photosynthesis of lichens: performance differences in species with green and blue–green algae as phycobionts. Oecologia 71:104–110
Logan JA (1985) Tropospheric ozone: seasonal behavior, trends, and anthropogenic influence. J Geophys Res 90:10463–10482
Lorenzini G, Landi U, Loppi S, Nali C (2003) Lichen distribution and bioindicator tobacco plants give discordant response: a case study from Italy. Environ Monit Assess 82:243–264
Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties od isoprene and its antioxidant role in leaves. Plant Physiol 126:993–1000
Matyssek R, Karnosky DF, Wieser G, Percy K, Oksanen E, Grams TEE, Kubiske M, Hanke D, Pretzsch H (2010) Advances in understanding ozone impact on forest trees: messages from novel phytotron and free–air fumigation studies. Environ Pollut 158:1990–2006
Mayaba N, Beckett RP (2001) The effect of desiccation on the activities of antioxidant enzymes in lichens from habitats of contrasting water status. Symbiosis 31:113–121
Nali C, Pucciariello C, Mills G, Lorenzini G (2005) On the different sensitivity of white clover clones to ozone: physiological and biochemical parameters in a multivariate approach. Water Air Soil Pollut 164:137–153
Nardini A, Marchetto A, Tretiach M (2013) Water relation parameters of six Peltigera species correlate with their habitat preferences. Fungal Ecol 6:397–407
Nimis PL (2016) The lichens of Italy. A second annotated catalogue. EUT, Trieste
Nimis PL, Martellos S (2008) ITALIC—The Information System on Italian Lichens. Version 4.0. http://dbiodbs.univ.trieste.it
Ojanperä K, Pätsikkä E, Yläranta T (1998) Effects of low ozone exposure of spring wheat on net CO2 uptake, Rubisco, leaf senescence and grain filling. New Phytol 138:451–460
Pellegrini E, Francini A, Lorenzini G, Nali C (2011) PSII photochemistry and carboxylation efficiency in Liriodendron tulipifera under ozone exposure. Environ Exp Bot 70:217–226
Pellegrini E, Bertuzzi S, Candotto Carniel F, Lorenzini G, Nali C, Tretiach M (2014) Ozone tolerance in lichens: a possible explanation from biochemical to physiological level using Flavoparmelia caperata as test organism. J Plant Physiol 171:1514–1523
Piccotto M, Tretiach M (2010) Photosynthesis in chlorolichens: the influence of the habitat light regime. J Plant Res 123:763–775
Riddell J, Padgett PE, Nash TH III (2010) Responses of the lichen Ramalina menziesii Tayl. to ozone fumigations. Bibl Lichenol 105:113–123
Riddell J, Padgett PE, Nash TH III (2012) Physiological responses of lichens to factorial fumigations with nitric acid and ozone. Environ Pollut 170:202–210
Roháček K (2002) Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 40:13–29
Rosentreter R, Ahmadjian V (1977) Effect of ozone on the lichen Cladonia arbuscula and the Trebouxia phycobiont of Cladina stellaris. Bryologist 80:600–605
Scheidegger C, Schroeter B (1995) Effects of ozone fumigation on epiphytic macrolichens: ultrastructure, CO2 gas exchange and chlorophyll fluorescence. Environ Pollut 88:345–354
Sgherri CLM, Navari-Izzo F (1995) Sunflower seedlings subjected to increasing water deficit stress: oxidative stress and defence mechanisms. Physiol Plant 93:25–30
Shavnin S, Maurer S, Matyssek R, Bilger W, Scheidegger C (1999) The impact of ozone fumigation and fertilization on chlorophyll fluorescence of birch leaves (Betula pendula). Trees-Struct Funct 14:10–16
Shin R, Berg RH, Schachtman DP (2005) Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol 46:1350–1357
Silberstein L, Siegel BZ, Siegel SM, Mukhtar A, Galun M (1996) Comparative studies on Xanthoria parietina, a pollution resistant lichen, and Ramalina duriaei, a sensitive species. II. Evaluation of possible air pollution–protection mechanisms. Lichenologist 28:367–383
Slavov C, Reus M, Holzwarth AR (2013) Two different mechanisms cooperate in the desiccation-induced excited state quenching in Parmelia lichen. J Phys Chem B 117:11326–11336
Tretiach M, Piccotto M, Baruffo L (2007) Effects of ambient NOx on modulated chlorophyll a fluorescence in transplanted Flavoparmelia caperata (lichen). Environ Sci Technol 41:2978–2984
Tretiach M, Pavanetto S, Pittao E, Sanità di Toppi L, Piccotto M (2012a) Water availability modifies tolerance to photo–oxidative pollutants in transplants of the lichen Flavoparmelia caperata. Oecologia 168:589–599
Tretiach M, Baruffo L, Piccotto M (2012b) Effects of Mediterranean summer conditions on chlorophyll a fluorescence emission in the epiphytic lichen Flavoparmelia soredians: a field study. Plant Biosyst 146(suppl. 1):171–180
Vainonen JP, Kangasjärvi J (2015) Plant signalling in acute ozone exposure. Plant Cell Environ 38:240–252
Valencia-Islas N, Zambrano A, Rojas JL (2007) Ozone reactivity and free radical scavenging behaviour of phenolic secondary metabolites in lichens exposed to chronic oxidant air pollution from Mexico City. J Chem Ecol 33:1619–1634
van Goethem TMWJ, Azevedo LB, van Zelm R, Hayes F, Ashmore MR, Huijbregts AJ (2013) Plant species sensitivity distribution for ozone exposure. Environ Pollut 178:1–6
Veerman J, Vasil’ev S, Paton GD, Ramanauskas J, Bruce D (2007) Photoprotection in the lichen Parmelia sulcata: the origins of desiccation–induced fluorescence quenching. Plant Physiol 145:997–1005
Vráblíková H, Barták M, Wonisch A (2005) Changes in glutathione and xanthophyll cycle pigments in the high light–stressed lichens Umbilicaria antarctica and Lasallia pustulata. J Photochem Photobiol B Biol 35–41
Wang S, Jiao HJ, Faust M (1991) Changes in ascorbate, glutathione and related enzyme activities during thidiazuron–induced bud break apple. Physiol Plant 82:231–236
Weissmann L, Garty J, Hochman A (2005a) Rehydration of the lichen Ramalina lacera results in production of reactive oxygen species and nitric oxide and a decrease in antioxidants. Appl Environ Microbiol 71:2121–2129
Weissmann L, Garty J, Hochman A (2005b) Characterization of enzymatic antioxidants in the lichen Ramalina lacera and their response to rehydration. Appl Environ Microbiol 71:6508–6514
Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjärvi J, Sandermann H, Langebartels C (2002) Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ 25:717–726
Wu S, Mickley LJ, Jacob DJ, Rind D, Streets DG (2008) Effects of 2000–2050 changes in climate and emissions on global tropospheric ozone and the policy–relevant background ozone in the United States. J Geophys Res 113:D06302
Yamamoto M, Kinoshita Y, Yoshimura I (2002) Photobiont culturing. In: Kranner I, Beckett RP, Varma AK (eds) Protocols in lichenology. Culturing, biochemistry, ecophysiology and use in biomonitoring. Springer, Berlin–Heidelberg, pp 34–42
Zhang J, Kirkham MB (1994) Drought–stress–induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:783–791
Acknowledgments
Thanks are due to M. Buiatti, E. Clagnan, T. Craighero and D. Kodnik (Trieste) for lichen processing. This study was published in the framework of the PRIN 2008 project “Lichens as biomonitors of photochemical pollutants” (nat. resp. M. Tretiach) and the PRIN 2010-11 project “Planning the green city in the global change era: urban tree functions and suitability for predicted future climates” (nat. resp. G. Lorenzini), funded by the Italian Ministry of Education, University, and Research.
Author information
Authors and Affiliations
Contributions
All authors took part to the experimental design development. EP and SB performed, respectively, the biochemical analysis and the Chl a F measurements, whereas EP, SB, FCC and GI performed the data analysis. MT, SB, EP, FCC and GI wrote the manuscript. GL, CN and MT are the project supervisors: they contributed actively with their expertise in each step of the work, and co-edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOC 389 kb).
Rights and permissions
About this article
Cite this article
Bertuzzi, S., Pellegrini, E., Candotto Carniel, F. et al. Ozone and desiccation tolerance in chlorolichens are intimately connected: a case study based on two species with different ecology. Environ Sci Pollut Res 25, 8089–8103 (2018). https://doi.org/10.1007/s11356-017-9444-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9444-0