Abstract
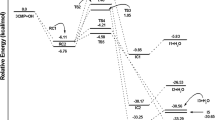
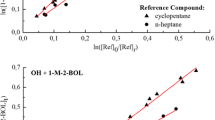
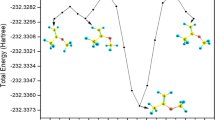
As the result of biogenic and anthropogenic activities, large quantities of chemical compounds are emitted into the troposphere. Alkanes, in general, and cycloalkanes are an important chemical class of hydrocarbons found in diesel, jet and gasoline, vehicle exhaust emissions, and ambient air in urban areas. In general, the primary atmospheric fate of organic compounds in the gas phase is the reaction with hydroxyl radicals (OH). The oxidation by Cl atoms has gained importance in the study of atmospheric reactions because they may exert some influence in the boundary layer, particularly in marine and coastal environments, and in the Arctic troposphere. The aim of this paper is to study of the atmospheric reactivity of methylcylohexanes with Cl atoms and OH radicals under atmospheric conditions (in air at room temperature and pressure). Relative kinetic techniques have been used to determine the rate coefficients for the reaction of Cl atoms and OH radicals with methylcyclohexane, cis-1,4-dimethylcyclohexane, trans-1,4-dimethylcyclohexane, and 1,3,5-trimethylcyclohexane at 298 ± 2 K and 720 ± 5 Torr of air by Fourier transform infrared) spectroscopy and gas chromatography–mass spectrometry (GC-MS) in two atmospheric simulation chambers. The products formed in the reaction under atmospheric conditions were investigated using a 200-L Teflon bag and employing the technique of solid-phase microextraction coupled to a GC-MS. The rate coefficients obtained for the reaction of Cl atoms with the studied compounds are the following ones (in units of 10−10 cm3 molecule−1 s−1): (3.11 ± 0.16), (2.89 ± 0.16), (2.89 ± 0.26), and (2.61 ± 0.42), respectively. For the reactions with OH radicals the determined rate coefficients are (in units of 10−11 cm3 molecule−1 s−1): (1.18 ± 0.12), (1.49 ± 0.16), (1.41 ± 0.15), and (1.77 ± 0.23), respectively. The reported error is twice the standard deviation. A detailed mechanism for ring-retaining product channels is proposed to justify the observed reaction products. The global tropospheric lifetimes estimated from the reported OH- and Cl-rate coefficients show that the main removal path for the investigated methylcyclohexanes is the reaction with OH radicals. But in marine environments, after sunrise, Cl reactions become more important in the tropospheric degradation. Thus, the estimated lifetimes range from 16 to 24 h for the reactions of the OH radical (calculated with [OH] = 106 atoms cm-3) and around 7–8 h in the reactions with Cl atoms in marine environments (calculated with [Cl] = 1.3 × 105 atoms cm-3). The reaction of Cl atoms and OH radicals and methylcylohexanes can proceed by H abstraction from the different positions.












Similar content being viewed by others
References
Alpendurada MD (2000) Solid-phase microextraction: a promising technique for sample preparation in environmental analysis. J Chromatogr A 889:3–14
Anderson RS, Huang L, Iannone R, Rudolph J (2007) Measurements of the C-12/C-13 kinetic isotope effects in the gas-phase reactions of light alkanes with chlorine atoms. J Phys Chem A 111:495–504
Anderson RS, Huang L, Iannone R, Thompson AE, Rudolph J (2004) Carbon kinetic isotope effects in the gas phase reactions of light alkanes and ethene with the OH radical at 296 ± 4 K. J Phys Chem A 108:11537–11544
Arthur CL, Pawliszyn J (1990) Solid-Phase microextraction with thermal-desorption using fused-silica optical fibers. Anal Chem 62:2145–2148
Aschmann SM, Atkinson R (1995) Rate constants for the gas-phase reactions of alkanes with Cl atoms at 296 ± 2 K. Int J Chem Kinet 27:613–622
Atkinson R (1986) Kinetics and mechanism of the gas-phase reactions of the hydroxyl radicals with organic compounds under atmospheric conditions. Chem Rev 86:69–201
Atkinson R (2003) Kinetics of the gas-phase reactions of OH radicals with alkanes and cycloalkanes. Atmos Chem Phys 3:2233–2307
Atkinson R, Arey J, Aschmann SM (2008) Atmospheric chemistry of alkanes: review and recent developments. Atmos Environ 42:5859–5871
Atkinson R, Carter WPL, Aschmann SM, Winer AM, Pitts JN (1984) Kinetics of the reaction of OH radicals with a series of branched alkanes at 297 ± 2 K. Int J Chem Kinet 16:469–481
Baker J, Arey J, Atkinson R (2004) Rate constants for the gas-phase reactions of OH radicals with a series of hydroxyaldehydes at 296 ± 2 K. Journal Of Physical Chemistry A 108:7032–7037
Ballesteros B, Ceacero-Vega AA, Garzon A, Jimenez E, Albaladejo J (2009) Kinetics and mechanism of the tropospheric reaction of tetrahydropyran with Cl atoms. J Photochem Photobiol A-Chem 208:186–194
Baulch DL, Bowers M, Malcolm DG, Tuckerman RT (1986) Evaluated kinetic data for high-temperature reactions—vol 5.1. Homogeneous gas-phase reactions of the hydroxyl radical with alkanes. J Phys Chem Ref Data 15:465–592
Baxley JS, Wells JR (1998) The hydroxyl radical reaction rate constant and atmospheric transformation products of 2-butanol and 2-pentanol. Int J Chem Kinet 30:745–752
Behnke W, Zetzsch C (1990) Heterogeneous photochemical rormation of Cl-atoms from NaCl aerosol, Nox and Ozone. J Aerosol Sci 21:S229–S232
Cavender AE, Biesenthal TA, Bottenheim JW, Shepson PB (2008) Volatile organic compound ratios as probes of halogen atom chemistry in the Arctic. Atmos Chem Phys 8:1737–1750
Ceacero-Vega AA, Ballesteros B, Albaladejo J, Bejan I, Barnes I (2009) Temperature dependence of the gas-phase reactions of Cl atoms with propene and 1-butene between 285 < T < 313 K. Chem Phys Lett 484:10–13
Ceacero-Vega AA, Ballesteros B, Bejan I, Barnes I, Albaladejo J (2011) Daytime reactions of 1,8-cineole in the troposphere. ChemPhysChem 12:2145–2154
Ceacero-Vega AA, Ballesteros B, Bejan I, Barnes I, Jimenez E, Albaladejo J (2012) Kinetics and mechanisms of the tropospheric reactions of menthol, borneol, fenchol, camphor, and fenchone with hydroxyl radicals (OH) and chlorine atoms (Cl). J Phys Chem A 116:4097–4107
DeMore WB, Bayes KD (1999) Rate constants for the reactions of hydroxyl radical with several alkanes, cycloalkanes, and dimethyl ether. J Phys Chem A 103:2649–2654
Harley RA, Cass GR (1995) Modeling the atmospheric concentrations of individual volatile organic-compounds. Atmos Environ 29:905–922
Hoekman SK (1992) Speciated measurements and calculated reactivities of vehicle exhaust emissions from conventional and reformulated gasolines. Environ Sci Technol 26:1206–1216
Jobson BT, Niki H, Yokouchi Y, Bottenheim J, Hopper F, Leaitch R (1994) Measurements of C-2–C-6 hydrocarbons during the polar sunrise 1992 experiment—evidence for Cl atom and Br atom chemistry. J Geophys Res-Atmos 99:25355–25368
Keil AD, Shepson PB (2006) Chlorine and bromine atom ratios in the springtime Arctic troposphere as determined from measurements of halogenated volatile organic compounds. J Geophys Res 111
Kramp F, Paulson SE (1998) On the uncertainties in the rate coefficients for OH reactions with hydrocarbons, and the rate coefficients of the 1,3,5-trimethylbenzene and m-xylene reactions with OH radicals in the gas phase. J Phys Chem A 102:2685–2690
Lawrence MG, Jockel P, von Kuhlmann R (2001) What does the global mean OH concentration tell us? Atmos Chem Phys 1:37–49
Leach J, Blanch A, Bianchi AC (1999) Volatile organic compounds in an urban airborne environment adjacent to a municipal incinerator, waste collection centre and sewage treatment plant. Atmos Environ 33:4309–4325
Li ZJ, Pirasteh A (2006) Kinetic study of the reactions of atomic chlorine with several volatile organic compounds at 240–340 K. Int J Chem Kinet 38:386–398
Lonneman WA, Seila RL, Meeks SA (1986) Nonmethane organic composition in the Lincoln Tunnel. Environ Sci Technol 20:790–796
Meagher RJ, McIntosh ME, Hurley MD, Wallington TJ (1997) A kinetic study of the reaction of chlorine and fluorine atoms with HC(O)F at 295 ± 2 K. Int J Chem Kinet 29:619–625
Olson KL, Sinkevitch RM, Sloane TM (1992) Speciation and quantitation of hydrocarbons in gasoline-engine exhaust. J Chromatogr Sci 30:500–508
Orlando JJ, Iraci LT, Tyndall GS (2000) Chemistry of the cyclopentoxy and cyclohexoxy radicals at subambient temperatures. J Phys Chem A 104:5072–5079
Orzechowska GE, Paulson SE (2005) Photochemical sources of organic acids. 1. Reaction of ozone with isoprene, propene, and 2-butenes under dry and humid conditions using SPME. Journal Of Physical Chemistry A 109:5358–5365
Oum KW, Lakin MJ, DeHaan DO, Brauers T, Finlayson-Pitts BJ (1998) Formation of molecular chlorine from the photolysis of ozone and aqueous sea-salt particles. Science 279:74–77
Ragains ML, Finlayson-Pitts BJ (1997) Kinetics and mechanism of the reaction of Cl atoms with 2-methyl-1,3-butadiene (isoprene) at 298 K. J Phys Chem A 101:1509–1517
Reisen F, Aschmann SM, Atkinson R, Arey J (2005) 1,4-hydroxycarbonyl products of the OH radical initiated reactions of C-5–C-8 n-alkanes in the presence of NO. Environ Sci Technol 39:4447–4453
Rowley DM, Lesclaux R, Lightfoot PD, Noziere B, Wallington TJ, Hurley MD (1992) Kinetic and mechanistic studies of the reactions of cyclopentylperoxy and cyclohexylperoxy radicals with HO2. J Phys Chem 96:4889–4894
Singh HB, Kasting JF (1988) Chlorine-hydrocarbon photochemistry in the marine troposphere and lower stratosphere. J Atmos Chem 7:261–285
Singh HB, Thakur AN, Chen YE, Kanakidou M (1996) Tetrachloroethylene as an indicator of low Cl atom concentrations in the troposphere. Geophys Res Lett 23:1529–1532
Smith DF, McIver CD, Kleindienst TE (1995) Kinetics and mechanism of the atmospheric oxidation of tertiary amyl methyl-ether. Int J Chem Kinet 27:453–472
Spicer CW, Chapman EG, Finlayson-Pitts BJ, Plastridge RA, Hubbe JM, Fast JD, Berkowitz CM (1998) Unexpectedly high concentrations of molecular chlorine in coastal air. Nature 394:353–356
Sprengnether MM, Demerjian KL, Dransfield TJ, Clarke JS, Anderson JG, Donahue NM (2009) Rate constants of nine C6–C9 alkanes with OH from 230 to 379 K: chemical Tracers for [OH]. J Phys Chem A 113:5030–5038
Treves K, Rudich Y (2003) The atmospheric fate of C-3–C-6 hydroxyalkyl nitrates. Journal Of Physical Chemistry A 107:7809–7817
Villanueva F, Barnes I, Monedero E, Salgado S, Gomez MV, Martin P (2007) Primary product distribution from the Cl-atom initiated atmospheric degradation of furan: environmental implications. Atmos Environ 41:8796–8810
Wilson EW, Hamilton WA, Kennington HR, Evans B, Scott NW, DeMore WB (2006) Measurement and estimation of rate constants for the reactions of hydroxyl radical with several alkanes and cycloalkanes. J Phys Chem A 110:3593–3604
Wingenter OW, Blake DR, Blake NJ, Sive BC, Rowland FS, Atlas E, Flocke F (1999) Tropospheric hydroxyl and atomic chlorine concentrations, and mixing timescales determined from hydrocarbon and halocarbon measurements made over the Southern Ocean. J Geophys Res-Atmos 104:21819–21828
Wingenter OW, Kubo MK, Blake NJ, Smith TW, Blake DR, Rowland FS (1996) Hydrocarbon and halocarbon measurements as photochemical and dynamical indicators of atmospheric hydroxyl, atomic chlorine, and vertical mixing obtained during Lagrangian flights. J Geophys Res-Atmos 101:4331–4340
Zweidinger RB, Sigsby JE, Tejada SB, Stump FD, Dropkin DL, Ray WD, Duncan JW (1988) Detailed hydrocarbon and aldehyde mobile source emissions from roadway studies. Environ Sci Technol 22:956–962
Acknowledgments
The authors would like to thank the Spanish Ministerio de Ciencia e Innovacion (Projects CGL2007-61835 and CGL2010-19066) and the Junta de Comunidades de Castilla-La Mancha (Projects PAI-05-062 and PCI08-0123-0381) for the financial support of this research work. A. A. Ceacero-Vega wishes to thank the first institution for providing him a grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
Rights and permissions
About this article
Cite this article
Ballesteros, B., Ceacero-Vega, A.A., Jiménez, E. et al. Atmospheric reactions of methylcyclohexanes with Cl atoms and OH radicals: determination of rate coefficients and degradation products. Environ Sci Pollut Res 22, 4806–4819 (2015). https://doi.org/10.1007/s11356-014-2901-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2901-0