Abstract
Purpose
Models of appetite control have been largely based on negative feedback from gut and adipose signaling to central appetite centers. However, contemporary models posit that fat-free mass (FFM) or the energy demand of FFM [i.e., resting metabolic rate (RMR)] may play a primary role in the motivational drive for food intake (i.e., food reinforcement). The relative reinforcing value of food (RRVfood) is associated with energy intake (EI) and increases with an acute energy deficit. Chronic exercise-induced energy deficits lead to alterations in fat mass (FM), FFM, and RMR and provide an opportunity to test whether change in (∆) FM, ∆FFM, ∆usual EI, or ∆RMR are associated with ∆RRVfood.
Methods
Participants (n = 29, BMI = 25–35 kg/m2) engaged in aerobic exercise expending 300 or 600 kcal, 5 days/weeks for 12 weeks. The reinforcing value of food (PMaxfood) was measured via a computer-based operant responding task and RRVfood was calculated as the reinforcing value of food relative to non-eating sedentary behaviors. RMR was determined by indirect calorimetry and body composition by DXA.
Results
Post-training FFM correlated with usual post-training EI (rs = 0.41, p < 0.05), PMaxfood (rs=0.52, p < 0.01), and RMR (rs = 0.85, p < 0.0001). ∆RMR negatively correlated with ∆PMaxfood (rs = − 0.38, p < 0.05) and with ∆RRVfood (rs = − 0.37, p < 0.05). ∆PMaxfood and ∆RRVfood were not associated with ∆FFM (p = 0.71, p = 0.57, respectively).
Conclusions
Reductions in RMR with weight loss may increase food reinforcement as means of restoring FFM and RMR to pre-weight loss amounts. Limiting reductions in RMR during weight loss may benefit weight maintenance by restricting increases in food reinforcement after weight loss.
Similar content being viewed by others
Introduction
Common models of body weight regulation propose that energy intake is loosely controlled via negative feedback loops [1]. For example, feedback from homeostatic gut peptides (e.g., ghrelin, cholecystokinin) signals acute changes in energy intake. Meanwhile, phasic signals from leptin and other adipokines provide tonic feedback as a consequence of adipose reserves [1]. These mechanisms can be problematic during weight loss regimens as they oppose loss of body mass. Additional complications arise when attempting to reduce fat mass (FM), as fat-free mass (FFM) can also be reduced during weight loss [2]. Both FM and FFM are consequential for body weight regulation as both tissues contribute to resting metabolic rate (RMR) [3, 4], accounting for 65–90% of total energy expenditure [5, 6]. FFM in particular is important for body weight control as it has a much greater energy demand than FM [7], accounting for 70–80% of RMR [8]. Blundell and colleagues have posited that appetite regulation and control of energy intake goes beyond negative feedback and lipostatic mechanisms to also include the energetic demands of RMR [9, 10]. They propose the tonic effects of FFM on energy intake are mediated through RMR, and that FFM may exert an ‘active’ drive for food. Moreover, FFM is protective against cardiometabolic disease [11, 12], and as such may be regulated resulting in an increased ‘active’ drive to eat after weight loss to replenish FFM to pre-weight loss levels, even if this is accompanied by simultaneous gains in FM [9, 10].
A validated approach to conceptualizing the ‘active’ drive for food is the relative reinforcing value of food (RRVfood) measured as the amount of operant responding, or effort a person is willing to complete to gain access to a food reinforcer relative to an alternative non-food reinforcer [13, 14]. RRVfood is associated with greater energy intake and obesity [15], and increases with acute (≤ 24 h) food deprivation [16]. The effect of body composition changes on RRVfood is not yet known. Increased RRVfood following weight loss could act as a compensatory response to return FFM to pre-weight loss amounts by increasing the ‘active’ drive for food. Moreover, if FFM or RMR are regulating food reinforcement after weight loss, then weight loss-induced reductions in FFM or RMR should be associated with increases in food reinforcement, but this has not yet been studied. Though exercise-induced weight loss is proposed to influence energy intake to a lesser degree than energy restriction [10], it provides a stronger test of the association between changes in FFM and RRVfood because any observed changes in RRVfood are not due to, nor confounded by, energy restriction or food deprivation [16]. Therefore, the purpose of this study was to test the hypothesis that changes in FFM or RMR with exercise training correlates to changes in food reinforcement, thus influencing the ‘active’ drive for food intake as measured by RRVfood.
Methods
Study design
This work is a pre–post study design to test a secondary hypothesis from a previous study that tested differences in energy intake, food reinforcement, and circulating ghrelin between 3000 kcal/week and 1500 kcal/week of aerobic exercise energy expenditure. The full methods, assessments, and analyses are published elsewhere [17]. For the purposes of the current study, results are not presented by treatment group as those data are already published and discussed [17]. The trial is registered with ClinicalTrials.gov identifier NCT02152501. All outcomes were measured at baseline and after 12 weeks of aerobic exercise training.
Participants
Thirty-six overweight or obese (BMI 25–35 kg/m2) participants (26 women) were originally recruited for the study and were randomized to groups of 3000 kcal/week or 1500 kcal/week of aerobic exercise energy expenditure. Twenty-nine participants (21 female) completed the study, six (5 female) withdrew for personal reasons, and one was dismissed for noncompliance. Participants were not regularly exercising more than twice per week prior to study initiation, were non-smokers, and were not on medications that affected hunger or metabolism. All participants were recruited from the Grand Forks ND metropolitan area. Appropriateness of exercise was screened using a Health History and Physical Activity Readiness Questionnaire (PAR-Q). All procedures were approved by the University of North Dakota Institutional Review Board, and participants provided written informed consent. Data were collected from April to October, 2016.
Exercise intervention
Exercise was prescribed to induce either 1500 or 3000 kcal expenditure per week. Exercise could be completed on a treadmill, elliptical ergometer, or cycle ergometer. Participants expended either 300 or 600 kcal per day, 5 days a week, for 12 weeks. Vivofit activity trackers (Garmin, Kansas City, KS) were provided for all participants to monitor compliance. Dietary recommendations were not discussed as part of the intervention.
Outcome measures
Body composition
FM, FFM, and % body fat were determined via dual X-ray absorptiometry (DXA) using a GE Lunar iDXA machine, and scans were analyzed using the GE Lunar enCORE software (GE Medical Systems, Madison, WI). Full body scans were conducted with participants lying supine on the table with arms to their side. Scans were taken in normal scan mode unless the software suggested “thick” scan mode. Automatic edge detection was used for scan analyses, and the machine was calibrated each day of use with the GE Lunar calibration phantom.
Resting metabolic rate
RMR was determined using indirect calorimetry. Participants traveled via automobile to the Grand Forks Human Nutrition Center ≥ 10 h after eating and ≥ 48 h after exercise. The equipment was calibrated prior to each assessment using a 3.0 L syringe for the flowmeter and known gas concentrations for the gas analyzer. Upon arrival, participants completed a questionnaire to ensure compliance. For the assessment, participants laid supine in a dimly lit, temperature-controlled room for 30 min, followed by a 30 min RMR measurement using a TrueOne 2400 metabolic cart (Parvo Medics, Sandy, UT). Participants were monitored to verify they stayed awake throughout the assessment, and that fractional expired CO2 was between 0.8 and 1.2%. The criterion for a valid RMR was 15 min of steady state, determined as < 10% fluctuation in VO2 and < 5% fluctuation in respiratory quotient (RQ). The Weir equation (46) was used to determine RMR from the measured VO2 and VCO2. These methods are validated and have been previously published [17, 18].
Food reinforcement
The reinforcing value of food (RRVfood) was assessed using previously described methods [17, 19]. A computer task assessed the amount of operant responding a participant was willing to perform to earn a portion of their favorite test food (Doritos, Snickers, Chips Ahoy cookies, powdered donuts, or Oreo cookies) relative to the amount of operant responses to earn access to their most-liked, sedentary behavior (time for activities such as watching TV, reading magazines, or completing crossword or Sudoku puzzles). Participants were given free choice to work at two proximal computer stations. The first station allowed the participant to earn an allotment of their most-liked test food and the other station allowed them to earn access to sedentary, non-eating behavior. The operant responses were measured in presses of a mouse button using a computer program mimicking a slot machine. Each click of the mouse changed three images on the screen, and a point was earned each time the slot machine produces three matching images. The slot machine awarded points based on operant responses, where clicks required to earn points doubled with each completed schedule of reinforcement. A schedule was completed for every five points earned (beginning with 4 clicks per point for schedule 1, 8 clicks per point for schedule 2, 16 clicks for schedule 3, etc.), and each completed schedule was awarded either a 100 kcal portion of the chosen snack food, or 5 min of sedentary activity. For example, it would initially require four mouse clicks to earn a point in schedule 1. After completion of schedule 1 (20 total clicks), participants had earned either a 100 kcal portion of food, or 5 min of sedentary activity time, and the game then required eight clicks to earn a point for schedule 2. After five points were earned in schedule 2, rewards would increase, and participants moved in to schedule 3, and so on. Each participant was free to earn as much or as little of each reinforcer as they desired (play the game as long as they wished), switch between stations as they desired, and were given immediate access to the reinforcers earned upon voluntary conclusion of the assessment. Outcome measures for food reinforcement included the greatest schedule completed to earn food (PMaxfood). RRVfood was calculated as [PMaxfood/(PMaxfood + PMaxsed)].
Energy intake
Dietary intake was assessed using the online version of the automated self-administered 24 h dietary recall (ASA24), which estimated total energy intake (EI), grams of carbohydrate, fat, protein and alcohol for 2 week days and one weekend day during the exercise intervention [20]. Participants are guided through multiple passes of dietary intake, and visual aids are provided to improve portion size estimations [21].
Analytic plan
Changes in outcome measures from pre- to post-intervention (∆) were determined by subtracting the baseline measurement from the post-training measurement. PMax and RRV values were rank-order variables, therefore, Spearman correlations were used to determine relationships between variables at pre, post, and between ∆ scores, to account for multiple comparisons, correlation p values were adjusted using the Benjamini–Hochberg procedure. After setting the false discovery rate at 20%, p values less than 0.05 were deemed significant [22]. Wilcoxon tests were used to determine differences between pre- and post-training for PMax and RRV data. Energy intake, fat mass, fat-free mass, and resting metabolic rate are continuous variables, therefore, students t tests were used to determine differences between pre- and post-training for these outcomes. To account for multiple comparisons in t tests, alpha (0.05) was divided by 8 (to account for variables: RRV, PMax, RMR, EI, weight, FM, FFM, %fat), requiring a p < 0.0063 to indicate significance. SAS version 9.4 (SAS Institute, Cary, NC) was used to fit the models using the CORR, or GLIMMIX, procedure. A post hoc power analysis [23] revealed 96% power to detect a 10% change when using the sample size, standard deviations, and mean changes associated with the PMaxfood outcome data in the current study. Figures were created using GraphPad Prism 9.
Results
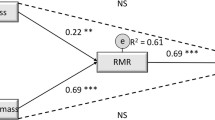
Overall, PMaxfood decreased (p < 0.0063) from baseline to post-exercise training, while RRVfood, EI, FM, FFM, and RMR were not different (p = 0.95, p = 0.04, p = 0.22, p = 0.47, p = 0.31, respectively) (Table 1). Considering the marked variation and bidirectional participant responses to exercise training, further analysis primarily focused on the following correlational data. At baseline (Table 2) RMR correlated with FM (rs = 0.34, p < 0.05; Fig. 1A), and FFM (rs = 0.87, p < 0.0001; Fig. 1B). Baseline RRVfood was correlated with usual EI (rs = 0.41, p < 0.05, Fig. 1C). Baseline PMaxfood and RRVfood were not associated with baseline RMR, FFM, or FM, and FFM was not associated with EI. After 12 weeks of aerobic exercise training (Table 3), RMR was no longer correlated with FM (rs = 0.25, p = 0.19), but remained correlated with FFM (rs = 0.85, p < 0.0001; Fig. 2A). Also, post-training PMaxfood was correlated with FFM (rs = 0.52, p < 0.01; Fig. 2B) but not with EI, RMR or FM after training, and usual EI was correlated with FFM (rs = 0.41, p < 0.05; Fig. 2C). No other post-training measures were correlated.
Correlations among the change scores of the outcome variables are shown in Table 4. ∆RMR was negatively correlated with ∆PMaxfood (rs = − 0.38, p < 0.05; Fig. 3A) and ∆RRVfood (rs = − 0.37, p < 0.05; Fig. 3B). Interestingly, neither ∆FM nor ∆FFM significantly correlated with ∆PMaxfood or ∆RRVfood. No other significant correlations were observed between changes in outcome measures, including ∆FFM vs. ∆RMR (rs = 0.06, p = 0.73).
Discussion
The primary findings from the current study show that ∆RMR is negatively correlated with ∆RRVfood and ∆PMaxfood, suggesting that if RMR changes as a result of exercise training, individuals may experience an inverse change in their active drive for food. Although FFM correlated with PMaxfood post-training, neither ∆RRVfood nor ∆PMaxfood were correlated with ∆FFM, which does not follow the proposed model of FFM determining the active drive for food and subsequent EI [9]. FFM certainly plays a major role in determining RMR, but these data suggest that role may not be independently sufficient to promote an increased drive for food following exercise-induced weight loss. Instead, ∆RMR inversely correlated to ∆RRVfood and ∆PMaxfood, suggesting that exercise-induced changes in RMR may promote an opposing change in the drive for food, potentially fostering a return to pre-intervention levels of FFM. For example, individuals with reduced RMR following exercise-induced weight loss would subsequently experience an increase in their active drive for food in an attempt to reestablish their pre-intervention RMR. These data encompass both positive and negative changes in RMR, suggesting an allostatic mechanism that promotes a return to pre-intervention RMR.
Prior studies support a mechanism by which FM is regained in excess while FFM returns to pre-weight loss levels in a phenomenon commonly known as “fat-overshooting” [24,25,26], which can lead to greater adiposity following weight loss interventions [27]. According to Dulloo and colleagues [28], this phenomenon results in an “increased drive to eat which persists beyond the point by which FM is restored”. The current data provide a potential mechanism that works in concert with these prior findings, however, stem from changes in RMR but not FFM.
It is important to note that there were no differences in FM, FFM or RMR between baseline and post-training in this analysis as we did not control for diet. Participants widely varied in their compositional responses to the training protocol, with some showing positive, and others negative ∆FFM and ∆RMR. Exercise training can lead to either an increased, or decreased FFM [29] depending on energy intake and dietary choices, as was exhibited by participants in this study. Therefore, the correlational data are a primary focal point of the analysis as it provides insight into bidirectional changes in RMR and FFM. Notably, no correlation was observed between ∆FFM and ∆RMR, suggesting that ∆RMR can stem from factors other than ∆FFM. These factors may include metabolic compensation/adaptive thermogenesis [30], the thermogenic effect of feeding (as diet was uncontrolled in this study), and by changes in physical activity.
As shown in Table 1, participants decreased PMaxfood from baseline to post-training. As these previously sedentary, overweight/obese adults were enrolled in an exercise training study, the increased awareness of dietary choices likely contributed to the observed decrease in Pmaxfood for these participants [31]. It is unexpected that PMaxfood would remain reduced considering the propensity for weight regain following weight loss [32]. Further research is needed to determine the duration that PMaxfood is reduced following exercise-induced weight loss and how to most effectively reduce PMaxfood with an exercise program, which would be expected to curb energy intake and prove beneficial in maintaining weight loss.
At baseline, RMR was positively correlated with both FFM and FM. Both FFM and FM contribute to RMR as metabolically active tissues, but contributions from FFM are far greater than FM (see review, [5]). Consistent with prior food reinforcement studies, baseline RRVfood was positively correlated with EI [14, 15, 33, 34]. Considering that RRVfood is a measure of the motivational drive to access food, these baseline results are in the expected directions and provide evidence for the validity of the RMR, body composition, food reinforcement, and EI assessment measures.
After training, FFM maintained a strong positive association with RMR further supporting FFM as a primary determinant of RMR [5, 7, 35]. Though not correlated at baseline, FFM was positively correlated with EI after training which suggests that the role of FFM in influencing EI [36, 37] may be uncovered after exercise-induced weight loss. FFM correlated with PMaxfood, EI, and RMR following exercise-induced weight loss, suggesting that FFM contributes to RMR, the drive for food, and ultimately increased EI [9]. FFM may, therefore, influence the drive for food indirectly, via its contribution to RMR.
The nonsignificant correlation between FM and RMR after exercise-induced weight loss suggests that the percentage of RMR determined by FM is reduced after training. Studies show conflicting reports of the association of FM with RMR, which has been attributed to either the small energy contribution of FM relative to FFM, or that the energy expenditure from FM contributing to RMR is relatively insignificant until individuals reach a state of obesity [5].
Importantly, this study has strengths and weaknesses that should not be overlooked. As this was a secondary study assessing a novel research question, methodologic precautions were taken to avoid type I statistical error by adjusting alpha for multiple comparisons, and setting the false discovery rate at 20% for correlational analyses. An effort to avoid type II statistical error was accomplished using a dataset with high statistical power for the given outcome measures (see Analytic plan). Regarding weaknesses, the sample population was recruited with the original research question in mind. Additionally, the correlations demonstrated in this paper do not prove causation, but do support the relationships that we have described above. Future research is needed to verify any cause-effect directionality, and validate the potential relationships identified in this secondary data analysis.
Conclusion
Responses in RRVfood following exercise training may be an allostatic response to reestablish pre-intervention RMR. The current data suggest that exercise-induced changes in RMR may drive an inverse change in the active drive for food, potentially promoting a return to pre-intervention RMR. Further, negative ∆RMR as a result of weight loss was associated with the active drive for food independent of ∆FFM. Therefore, limiting reductions in RMR during weight loss may benefit weight loss maintenance by limiting increases in RRVfood. Further studies are warranted to confirm the potential relationships identified in this study, and substantiate any possible cause–effect relationships.
Data availability
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
Code availability
Not applicable.
Abbreviations
- FFM:
-
Fat-free mass
- RRVfood :
-
Relative reinforcing value of food
- PMaxfood :
-
Reinforcing value of food
- RMR:
-
Resting metabolic rate
- DXA:
-
Dual X-ray absorptiometry
- EI:
-
Energy intake
- Δ:
-
Changes in
References
Woods SC, Ramsay DS (2011) Food intake, metabolism and homeostasis. Physiol Behav 104(1):4–7
Chaston T, Dixon J, O’brien P (2007) Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes 31(5):743–750
Leibel RL, Rosenbaum M, Hirsch J (1995) Changes in energy expenditure resulting from altered body weight. N Engl J Med 332(10):621–628
Weinsier RL, Hunter GR, Zuckerman PA, Redden DT, Darnell BE, Larson DE, Newcomer BR, Goran MI (2000) Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr 71(5):1138–1146
Cunningham JJ (1991) Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr 54(6):963–969. https://doi.org/10.1093/ajcn/54.6.963
Elia M (1992) Energy expenditure in the whole body. Energy metabolism: tissue determinants and cellular corollaries:19–59
Nelson KM, Weinsier RL, Long CL, Schutz Y (1992) Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr 56(5):848–856. https://doi.org/10.1093/ajcn/56.5.848
Sparti A, DeLany JP, de la Bretonne JA, Sander GE, Bray GA (1997) Relationship between resting metabolic rate and the composition of the fat-free mass. Metabolism 46(10):1225–1230. https://doi.org/10.1016/S0026-0495(97)90222-5
Blundell FG, Gibbons C, Caudwell P, Hopkins M (2015) The biology of appetite control: do resting metabolic rate and fat-free mass drive energy intake? Physiol Behav 152:473–478. https://doi.org/10.1016/j.physbeh.2015.05.031
Stubbs RJ, Hopkins M, Finlayson G, Duarte C, Gibbons C, Blundell J (2018) Potential effects of fat mass and fat-free mass on energy intake in different states of energy balance. Eur J Clin Nutr 72(5):698
Bosy-Westphal A, Braun W, Geisler C, Norman K, Muller MJ (2018) Body composition and cardiometabolic health: the need for novel concepts. Eur J Clin Nutr 72(5):638–644. https://doi.org/10.1038/s41430-018-0158-2
Wells JCK (2017) Body composition and susceptibility to type 2 diabetes: an evolutionary perspective. Eur J Clin Nutr 71(7):881–889. https://doi.org/10.1038/ejcn.2017.31
Epstein LJJ (2006) Food reinforcement. Appetite 46(1):22–25. https://doi.org/10.1016/j.appet.2005.04.006
Saelens BE, Epstein LH (1996) Reinforcing value of food in obese and non-obese women. Appetite 27(1):41–50
Epstein CKA, Lin H, Fletcher KD (2011) Food reinforcement, energy intake, and macronutrient choice. Am J Clin Nutr 94(1):12–18. https://doi.org/10.3945/ajcn.110.010314
Raynor HA, Epstein LH (2003) The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite 40(1):15–24. https://doi.org/10.1016/S0195-6663(02)00161-7
Flack KD, Ufholz K, Johnson L, Fitzgerald JS, Roemmich JN (2018) Energy compensation in response to aerobic exercise training in overweight adults. Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/ajpregu.00071.2018
Flack S, Johnson R (2016) Cross-validation of recent and longstanding resting metabolic rate prediction equations. FASEB J. https://doi.org/10.1096/fasebj.30.1_supplement.682.4
Casperson SL, Johnson L, Roemmich JN (2017) The relative reinforcing value of sweet versus savory snack foods after consumption of sugar- or non-nutritive sweetened beverages. Appetite 112:143–149. https://doi.org/10.1016/j.appet.2017.01.028
McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC (2002) Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 76(6):1261–1271. https://doi.org/10.1093/ajcn/76.6.1261
Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, Willis G, Islam NG, Baranowski T, McNutt S, Potischman N (2012) The automated self-administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet 112(8):1134–1137. https://doi.org/10.1016/j.jand.2012.04.016
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Methodol) 57(1):289–300
Gerow KG (2007) Power and sample size estimation techniques for fisheries management: assessment and a new computational tool. North Am J Fish Manag 27(2):397–404. https://doi.org/10.1577/M05-094.1
Friedl KE, Moore RJ, Hoyt RW, Marchitelli LJ, Martinez-Lopez LE, Askew EW (2000) Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J Appl Physiol 88(5):1820–1830
Nindl B, Friedl K, Frykman P, Marchitelli L, Shippee R, Patton J (1997) Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int J Sports Med 18(05):317–324
Keys A, Brožek J, Henschel A, Mickelsen O, Taylor HL (1950) The biology of human starvation, vol 2. University of Minnesota Press
Weyer C, Walford RL, Harper IT, Milner M, MacCallum T, Tataranni PA, Ravussin E (2000) Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. Am J Clin Nutr 72(4):946–953. https://doi.org/10.1093/ajcn/72.4.946
Dulloo AG, Jacquet J, Miles-Chan JL, Schutz Y (2017) Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. Eur J Clin Nutr 71(3):353
Weinheimer EM, Sands LP, Campbell WW (2010) A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev 68(7):375–388. https://doi.org/10.1111/j.1753-4887.2010.00298.x
Rosenbaum M, Leibel RL (2010) Adaptive thermogenesis in humans. Int J Obes (Lond) 34(Suppl 1):S47-55. https://doi.org/10.1038/ijo.2010.184
Hahn SL, Linxwiler AN, Huynh T, Rose KL, Bauer KW, Sonneville KR (2021) Impacts of dietary self-monitoring via MyFitnessPal to undergraduate women: a qualitative study. Body Image 39:221–226. https://doi.org/10.1016/j.bodyim.2021.08.010
Garner DM, Wooley SC (1991) Confronting the failure of behavioral and dietary treatments for obesity. Clin Psychol Rev 11(6):729–780. https://doi.org/10.1016/0272-7358(91)90128-H
Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ (2007) Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci 121(5):877
Giesen JC, Havermans RC, Douven A, Tekelenburg M, Jansen A (2010) Will work for snack food: the association of BMI and snack reinforcement. Obesity 18(5):966–970
Weise CM, Hohenadel MG, Krakoff J, Votruba SB (2013) Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. Int J Obes 38:243. https://doi.org/10.1038/ijo.2013.85
Blundell CP, Gibbons C, Hopkins M, Näslund E, King NA, Finlayson G (2012) Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br J Nutr 107(3):445–449
Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Näslund E, Blundell JE (2012) Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr 97(1):7–14
Acknowledgements
This work was supported by the United States Department of Agriculture, Agricultural Research Service, project 3062-51000-051-00D
Funding
This study was funded by the United States Department of Agriculture—Agricultural Research Service, Project 3062-51000-051-00D.
Author information
Authors and Affiliations
Contributions
CLP: Lead author—Led study idea development, ran statistical analyses, and composed manuscript draft. KDF: Second author—Led study design and development, all aspects of recruitment, intervention management, and data collection. Assisted in manuscript revision. KEU: Third author—Assisted in data collection and revision of manuscript. LAJ: Fourth author—Statistician contributing to statistical analyses. JNR: Senior author—Led project development, study design, and responsible for funding. Assisted in manuscript revision and made final decisions on manuscript and data analysis. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All procedures were approved by the University of North Dakota Institutional Review Board. All study methods were performed in accordance with the standards set by the Helsinki declaration.
Consent to participate
All participants provided written informed consent.
Consent for publication
All participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pankey, C.L., Flack, K., Ufholz, K. et al. Influence of fat-free mass and resting metabolic rate on increased food reinforcement after exercise training. Sport Sci Health 18, 923–931 (2022). https://doi.org/10.1007/s11332-021-00876-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-021-00876-y