Abstract
Purpose
To compare the anatomical balance and shape of the upper airway in the supine position between adults with positional obstructive sleep apnea (POSA) and adults with non-positional OSA (NPOSA).
Methods
Adults diagnosed with OSA (apnea-hypopnea index (AHI) > 10 events/h) were assessed for eligibility. POSA was defined as the supine AHI more than twice the AHI in non-supine positions; otherwise, patients were classified as NPOSA. Cone beam computed tomography (CBCT) imaging was performed for every participant while awake in the supine position. The anatomical balance was calculated as the ratio of the tongue size to the maxillomandibular enclosure size. The upper airway shape was calculated as the ratio of the anteroposterior dimension to the lateral dimension at the location of the minimal cross-sectional area of the upper airway (CSAmin-shape).
Results
Of 47 participants (28 males, median age [interquartile range] 56 [46 to 63] years, median AHI 27.8 [15.0 to 33.8]), 34 participants were classified as having POSA (72%). The POSA group tended to have a higher proportion of males and a lower AHI than the NPOSA group (P = 0.07 and 0.07, respectively). After controlling for both sex and AHI, the anatomical balance and CSAmin-shape were not significantly different between both groups (P = 0.18 and 0.73, respectively).
Conclusion
Adults with POSA and adults with NPOSA have similar anatomical balance and shape of their upper airway in the supine position.
Trial registration
This study was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR Trial ACTRN12611000409976).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent complete (i.e., apnea) and partial (i.e., hypopnea) obstructions of the upper airway [1]. These respiratory events are most frequent and severe in the supine position [2]. It has been estimated that more than 50% of patients with OSA suffer from positional OSA (POSA) [3, 4]. Despite having well defined clinical features and specific treatment recommendations, the underlying mechanisms for POSA are not clear [5]. Evidence suggests that the differential pathogenesis between POSA and non-POSA (NPOSA) is partly attributable to anatomical factors, such as upper airway morphology [5, 6]. Some anatomical OSA treatments (e.g., upper airway surgery and oral appliance therapy) also show different treatment effects between patients with POSA and NPOSA [7, 8].
The upper airway is surrounded by soft tissue structures enclosed by maxillomandibular bony structures. Research suggests that both soft and hard craniofacial structures are related to upper airway shape and size, and these affect the severity of OSA [9]. The ratio of tongue size to maxillomandibular enclosure size – termed ‘anatomical balance’ – is an important risk factor in OSA, particularly in the supine position [10, 11]. Anatomical imbalance (i.e., an enlarged tongue size to maxillomandibular enclosure size ratio) indicates higher tissue pressure surrounding the upper airway and decreased upper airway size [12]. However, only a few studies have compared craniofacial structures between POSA and NPOSA groups. Two studies have suggested that patients with POSA may have a smaller lower facial height, more backward positioned mandible, and shorter soft palatal length than patients with NPOSA [13, 14]. However, Saigusa et al. [14] found no significant difference in the anatomical balance of the upper airway between the POSA and NPOSA groups in their study. Thus, studies on the differences in craniofacial structures between both groups are still limited.
Furthermore, studies by Pevernagie et al. [6] and Jiao et al. [13] have suggested that patients with NPOSA have a more circular cross-sectional area of the upper airway compared to patients with POSA, which may explain the differential pathogenesis between the groups. However, in both studies, participants had relatively severe OSA, and patients with NPOSA had more severe OSA. By contrast, a study by Joosten et al. [15] found no significant difference between POSA and NPOSA in upper airway shape, but the sample size was small (each group had only eight subjects). Hence, the difference in upper airway shape between POSA and NPOSA is not clear [5]. Accordingly, a large-scale study across the spectrum of OSA severity is necessary to provide more evidence on the potential differences in upper airway shape between POSA and NPOSA and their effect on treatment outcome.
In the supine position, the exacerbation of obstructive respiratory events may be related to the collapse of the anterior wall of the upper airway due to the effect of gravity. Accordingly, we hypothesize that: (1) adults with POSA have a greater anatomical imbalance (i.e., a higher ratio of tongue size to maxillomandibular enclosure size) compared to adults with NPOSA, and therefore a greater tendency of upper airway collapse in the anteroposterior direction in the supine position; and (2) adults with POSA have a more elliptically shaped cross-sectional area (i.e., a smaller ratio of the anteroposterior dimension to the lateral dimension) compared to adults with NPOSA, and therefore a greater tendency of upper airway collapse in the anteroposterior direction in the supine position. Accordingly, this study aimed to compare the anatomical balance and shape of the upper airway between adults with POSA and adults with NPOSA in the supine position.
Material and methods
Overview
This study is part of a prospective study [16], in which adults with OSA (apnea–hypopnea index (AHI) > 10 events/h) were recruited for building a prediction model for oral appliance treatment outcomes. This study received ethical approval (Sydney Local Health District, Protocol No. X11-0134 & HREC/11/RPAH/192), and informed consent was obtained from all participants. This study was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR Trial ACTRN12611000409976).
Participants
Patients were recruited prospectively from sleep clinics associated with a tertiary teaching hospital in Sydney (Royal North Shore Hospital). The inclusion criteria were adults (> 18 years of age) diagnosed with OSA (AHI > 10 events/h) using in-laboratory polysomnography (PSG) who were willing to try oral appliance therapy. Exclusion criteria were limited to contraindications to oral appliance therapy (e.g., severe periodontal disease and an insufficient number of teeth). There were no upper limits on AHI or body mass index (BMI) for recruitment. Ethnicity was recorded using a self-reporting questionnaire, in which participants selected their ethnicity based on their culture, religion, skin color, and language from the following four ethnic groups: Asian, Native Hawaiian and Pacific Islanders, Hispanic and Latino, and White. Patients were classified as positional OSA (POSA) if their AHI in supine position (AHI-supine) was more than twice their AHI in non-supine positions (AHI-non-supine) [17]. If this criterion was not fulfilled, patients were classified as non-positional OSA (NPOSA).
CBCT acquisition
Every participant underwent a CBCT scan (NewTom 3G, QR systems, Italy) in the supine position while awake [16]. During the scanning process, patients were asked to bite gently in natural occlusion, lightly touch their teeth with their tongue, refrain from swallowing, and bring their lips into a relaxed position. The exposure settings were 110 kVp, 2-3 mA, and exposure times were 5–15 s. Voxel size was 0.36 mm or 0.42 mm, depending on the scan settings. A standardization of head position was performed after scanning. During this process, the palatal plane was adjusted to be parallel to the axial and sagittal planes and perpendicular to the coronal plane. CBCT datasets were saved as Digital Imaging and Communications in Medicine (DICOM) files.
Anatomical balance
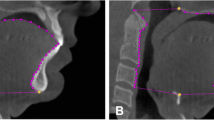
3Diagnosys® software (v5.3.1, 3diemme, Cantu, Italy) was used to measure craniofacial structures, including the soft palate, tongue, hyoid, maxilla, mandible, and face height. The primary variable – the anatomical balance of the upper airway – was calculated as the ratio of the tongue size to maxillomandibular enclosure size in the mid-sagittal plane of the CBCT image. Tongue size was defined as the area enclosed by the point Hyoid (H), Menton (Me), the contour of the frontal teeth and the tongue, and the base of the epiglottis (Fig. 1A). Meanwhile, the maxillomandibular enclosure size was defined as the area enclosed by the point Hyoid (H), Menton (Me), the contour of the front teeth, the hard palate, the point posterior nasal spine (PNS), and the anterior boundary of the second and third cervical vertebra (Fig. 1B). The definitions for the secondary outcome variables, such as the soft palate, tongue, hyoid, maxilla, mandible, and face height, are illustrated in Fig. 2.
Measurements of tongue size and maxillomandibular enclosure size using cone beam computed tomography (CBCT) imaging in the mid-sagittal plane. (A) Tongue size: area enclosed by the point Hyoid (H), Menton (Me), the contour of the frontal teeth and tongue, and the base of the epiglottis. (B) Maxillomandibular enclosure size: area enclosed by the point Hyoid (H), Menton (Me), the contour of the front teeth, the hard palate, point posterior nasal spine (PNS), and the anterior boundary of the second and third cervical vertebra
Illustration of the secondary outcome variables of the craniofacial structures. Soft palate length (distance from PNS to the tip of the soft palate), Hyoid to MP plane (distance between H and Mandibular plane), SNA (angle between points A and S at N), Maxillary length (distance between ANS and PNS), SNB (angle between points B and S at N), Mandibular length (distance between Me and Go), Mandibular plane angle (angle between Frankfort plane and Mandibular plane), Face height (distance between N and Me), Lower face height (distance between ANS and Me). Landmarks: N = nasion, S = sella, ANS = anterior nasal spine, PNS = posterior nasal spine, Go = gonion, Me = menton, H = hyoidale, A = subspinale, B = supramentale
Upper airway shape
Upper airway shape and size were analyzed using CBCT images with Amira® software (v4.1, Visage Imaging Inc., Carlsbad, CA, USA). The superior boundary of the upper airway was the palatal plane; the inferior boundary was the horizontal plane (parallel to the palatal plane) across the base of the epiglottis (Fig. 3A). After applying the upper airway boundaries, the total upper airway volume (V) and the cross-sectional area (CSA) of each slice were calculated using the same software. The minimum CSA (CSAmin) was identified based on the CSA results. The anteroposterior dimension (A-P) and lateral dimension (Lat) of CSAmin were measured (Fig. 3B) on the specific slice where the CSAmin was located. The shape of CSAmin (CSAmin-shape) was calculated as the ratio of A-P to Lat; this was the primary outcome variable for representing the upper airway shape at the CSAmin location.
Upper airway boundaries and measurements of the upper airway shape, using cone beam computed tomography (CBCT) imaging. (A) The segmentation of the upper airway from the upper boundary (U) to the lower boundary (L) in the mid-sagittal plane. (B) The measurement of the anterior–posterior dimension (A-P) and lateral dimension (Lat) of the minimum cross-sectional area of the upper airway (CSAmin) in the axial plane
Reliability of measurements
All upper airway variables were measured by an experienced examiner blinded to group membership. To determine the intra-observer reliability of the measurements, 15 CBCT scans were randomly selected and re-measured after three months of the original measurements.
The calculation of sample size for the reliability assessment was performed according to Walter et al. [18]. We assumed that our actual intra-observer reliability in the present study is at least 0.9, and a reliability of 0.6 or higher would be acceptable based on our previous study [19]. Therefore, the null hypothesis was defined as H0: ρ0 = 0.6, and the alternative hypothesis was defined as H1: ρ1 = 0.9, with a significance level of 0.05 and a power of 0.8. Based on this hypothesis, the proposed sample size was 15 patients [18].
Statistical analysis
A Shapiro-Wilk test was used to test the normality of continuous data. An independent t test (for normally distributed variables), a Mann–Whitney U test (for non-normally distributed variables), and a Chi-squared test (or Fisher’s exact test) (for nominal variables) were used to compare the baseline demographic characteristics and PSG parameters between the POSA and NPOSA groups.
An intraclass correlation coefficient (ICC) was performed to determine the intra-observer reliability of the measurements of anatomical variables. The anatomical variables of upper airway morphology were compared between the POSA and NPOSA groups using analysis of covariance (ANCOVA). The baseline characteristics that were significantly different between both groups were treated as covariates. The significance level was set at 0.05, and statistical analyses were carried out using SPSS software (SPSS version 26, Chicago, IL, USA).
The effect sizes for primary outcome variables were calculated using the program G*power (version 3.1.9, Franz Faul, Universität Kiel, Germany).
Results
Participant’s recruitment
A total of 60 patients were assessed for eligibility. Of these, 13 patients with incomplete information were excluded from the analysis: n = 2 with incomplete baseline demographic data, n = 5 with incomplete PSG data, and n = 6 with CBCT images that were distorted or/and lacked landmarks for the primary outcome variables. After exclusions, 47 patients were included in the analysis: 34 patients with POSA, and 13 patients with NPOSA.
Patient characteristics
The baseline demographic characteristics and respiratory and sleep parameters for POSA and NPOSA groups are provided in Table 1. There were no significant differences between the groups in age, sex, body mass index, neck circumference, waist circumference, and ethnicity (P = 0.07–0.88). For the respiratory variables, no significant differences were found in AHI and AHI-supine between both groups (P = 0.07 and 0.39, respectively); however, by definition, the AHI-non-supine was significantly higher in the NPOSA group compared to the POSA group (Z = −4.31, P < 0.01). For the sleep variables, there were no significant differences between the groups (P = 0.20–0.75). As the sex and AHI tended to have a significant difference between the groups (P = 0.07 and P = 0.07, respectively), these two variables were treated as covariates in the ANCOVA analyses.
Reliability of measurements
The intra-observer reliability was excellent for the primary variables (ICC = 0.91 for the anatomical balance, ICC = 0.97 for the CSAmin-shape) and secondary variables (ICC = 0.92–0.99) [20].
Anatomical balance
Table 2 provides the anatomical variables of the upper airway of the POSA and NPOSA groups. There was no significant difference between the groups for the primary outcome variable: the anatomical balance of the upper airway (F = 1.85, P = 0.18). The individual values of anatomical balance for both groups are shown in Fig. 4A. The effect size f of the anatomical balance comparison was 0.2 (partial η2 = 0.04, ANCOVA). This can be qualified as being between small and medium. For the secondary outcome variables, there were no significant differences between the groups either (all P > 0.05).
Upper airway shape
The upper airway dimensional variables of the POSA and NPOSA groups are shown in Table 3. For the primary outcome variable, CSAmin-shape, there was no significant difference between the groups (F = 0.12, P = 0.73). The individual values of the CSAmin-shape of both groups are presented in Fig. 4B. The effect size f of the CSAmin-shape comparison was 0.05 (partial η2 = 0.003, ANCOVA), which can be qualified as small. For the secondary outcome variables, there were no significant differences between the groups either (P = 0.23–0.98).
Discussion
In the present study, we hypothesized that adults with POSA have a greater anatomical imbalance and a more elliptically shaped cross-sectional area of the upper airway in the supine position compared to adults with NPOSA. The results of this study showed no significant difference between both groups in these outcomes.
Anatomical balance of the upper airway
No significant difference in the anatomical balance between the POSA and NPOSA groups was found. The observed effect size of the anatomical balance comparison was small to medium [21]. Therefore, the clinical relevance of the difference in this variable between both groups is controversial. Consistent with our results, Saigusa et al.’s study [14] calculated the anatomical balance using three-dimensional structures’ volume with magnetic resonance imaging (MRI) and found no significant difference between POSA and NPOSA either.
Regarding secondary variables, previous studies have indicated that patients with POSA show a larger ANB angle, smaller lower facial height, and shorter soft palate length compared to patients with NPOSA [13, 14]. However, we found no significant difference between both groups in those structures. Both previous studies’ samples were predominantly of Asian ethnicity, so the different ethnic balance in our study sample (74% white) may explain the different findings in these structures.
Upper airway shape
The present study found no significant difference in CSAmin-shape between the POSA and NPOSA groups. Furthermore, the observed effect size of the CSAmin-shape comparison was small [21], indicating that the difference in CSAmin-shape between both groups is not clinically relevant. In contrast to our study results, a study by Pevernagie et al. [6] indicates that CSAmin-shape was more circular in the NPOSA group compared to the POSA group in the supine position. In their study, however, the NPOSA group had significantly higher AHI than the POSA group (83 vs. 31 events/h). Since it has been suggested that CSAmin-shape is related to AHI [22], the higher AHI in the NPOSA group could be a confounding factor in the comparison of CSAmin-shape, and it may explain the different results in comparison to our study. In line with our results, a study by Joosten et al. [15] suggested no significant difference in CSAmin-shape between both groups. Although their study had a small sample size (each group had eight subjects), our study confirmed their results with a larger sample. Therefore, it seems that upper airway shape is similar for POSA and NPOSA groups in the supine position, especially when their AHI severity is matched.
Implications
As upper airway morphology was not significantly different between the POSA and NPOSA groups in the present study, it may be hypothesized that non-anatomical factors account for the differences in pathogenesis between both groups. Joosten et al. [23] have shown that, turning from a supine to a lateral position, the muscular ability to stiffen and dilate the airway is more effective in the POSA group compared to the NPOSA group. The less effective muscle activities in the NPOSA group may be caused by greater vulnerability to muscle fatigue (due to the increased number of type II fibers) [24], poor muscle effectiveness (caused by excessive fat or muscle hypertrophy) [25], and disturbed coordination of the neural drives between upper airway and respiratory muscles [26, 27]. Another study, undertaken by Joosten et al. [15], has suggested that lung volume decreases significantly in the POSA group when moving from the lateral to the supine position, while there is no significant change in lung volume with position changing in the NPOSA group. Decreased lung volume can increase upper airway collapsibility via caudal tracheal displacement [28, 29], which may be a trigger for upper airway collapse in the supine position in the POSA group [15]. However, these studies [15, 23] were performed either in only severe OSA samples or by comparing both groups with unmatched AHI. Therefore, whether POSA in comparison to NPOSA has its distinct pathophysiology characteristics still needs more research.
Limitations
This study has several limitations. First, the patients were awake during the CBCT examination, which may not accurately reflect upper airway morphology during sleep. However, taking CBCT images during sleep is quite challenging, therefore, upper airway imaging in the awake state has been widely used to study the underlying pathogenesis of OSA [30]. As the CBCT images were taken in the supine position for both groups in the present study, we assume that the comparison results are valid. However, sleep-induced changes in upper airway morphology may be different in both groups. Therefore, future research performed in the sleep state is needed to verify the current results. Second, the CBCT measurements were collected as part of a previous treatment study [16] and, hence, only taken in the supine position. Images in non-supine positions would show whether the current non-significant findings are also applicable in other sleeping positions. Future research, including scans in both positions, is therefore recommended. Moreover, as this study recruited patients from a prospective study [16], we did not perform an a priori power analysis to calculate the sample size. However, the effect size of the primary outcome variables indicates that the differences in the anatomical balance and the CSAmin-shape between both groups are not clinically relevant. Third, the present study used Cartwright’s definition [17] to classify POSA, which is the most commonly used definition to date. However, compared to other definitions [31,32,33,34], Cartwright’s definition is the most lenient one and can result in a higher prevalence of POSA [32, 35]. Therefore, our study possibly overestimated POSA, which may explain the relatively high prevalence of POSA (72%) and the non-significant findings. However, we did also test our results with the stricter definition of the Amsterdam Positional OSA Classification (APOC) [32]. Although some patients who did not have sufficient sleep time in supine and/or non-supine positions were excluded from the classification by using the APOC definition (reducing the total sample size to n = 38), the prevalence of POSA was similar (APOC vs Cartwright: 71% vs 72%), and the comparison results of the primary outcome variables were similar to the present ones. Therefore, the criteria used in the current study did not lead to biased results. POSA tends to be associated with mild and moderate OSA as opposed to severe OSA [31], and the majority of this study population (62%) was diagnosed with mild to moderate OSA, which may explain the high prevalence of POSA in our population.
Conclusions
Adults with POSA and adults with NPOSA have similar anatomical balance and shape of their upper airway in the supine position.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events: deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med 8(5):597–619
Oksenberg A, Arons E, Radwan H, Silverberg DS (1997) Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic and multiple sleep latency test data. Chest 112(3):629–639
Teerapraipruk B, Chirakalwasan N, Simon R, Hirunwiwatkul P, Jaimchariyatam N, Desudchit T, Charakorn N, Wanlapakorn C (2012) Clinical and polysomnographic data of positional sleep apnea and its predictors. Sleep Breath 16(4):1167–1172
Sabil A, Blanchard M, Trzepizur W, Goupil F, Meslier N, Paris A, Pigeanne T, Priou P, Le Vaillant M, Gagnadoux F (2020) Positional obstructive sleep apnea within a large multicenter French cohort: prevalence, characteristics, and treatment outcomes. J Clin Sleep Med 16(12):2037–2046. https://doi.org/10.5664/jcsm.8752
Joosten SA, O’Driscoll DM, Berger PJ, Hamilton GS (2014) Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev 18(1):7–17
Pevernagie DA, Stanson A, Sheedy P 2nd, Daniels BK, Shepard JW Jr (1995) Effects of body position on the upper airway of patients with obstructive sleep apnea. Am J Respir Crit Care Med 152(1):179–185
Lee CH, Kim S-W, Han K, Shin J-M, Hong S-L, Lee J-E, Rhee C-S, Kim J-W (2011) Effect of uvulopalatopharyngoplasty on positional dependency in obstructive sleep apnea. Arch Otolaryngol-Head Neck Surg 137(7):675–679
Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA (2015) Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med 11(8):861–868
Lee RW, Sutherland K, Cistulli PA (2010) Craniofacial morphology in obstructive sleep apnea: a review. Clin Pulm Med 17(4):189–195
Tsuiki S, Isono S, Ishikawa T, Yamashiro Y, Tatsumi K, Nishino T (2008) Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology 108(6):1009–1015. https://doi.org/10.1097/ALN.0b013e318173f103
Sutherland K, Almeida FR, de Chazal P, Cistulli PA (2018) Prediction in obstructive sleep apnoea: diagnosis, comorbidity risk, and treatment outcomes. Expert Rev Respir Med 12(4):293–307
ISONO S, (2012) Obesity and obstructive sleep apnoea: Mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology 17(1):32–42. https://doi.org/10.1111/j.1440-1843.2011.02093.x
Jiao X, Zou J, Liu S, Guan J, Yi H, Yin S (2017) A retrospective study: does upper airway morphology differ between non-positional and positional obstructive sleep apnea? PeerJ 5:e3918
Saigusa H, Suzuki M, Higurashi N, Kodera K (2009) Three-dimensional morphological analyses of positional dependence in patients with obstructive sleep apnea syndrome. Anesthesiology 110(4):885–890
Joosten SA, Sands SA, Edwards BA, Hamza K, Turton A, Lau KK, Crossett M, Berger PJ, Hamilton GS (2015) Evaluation of the role of lung volume and airway size and shape in supine-predominant obstructive sleep apnoea patients. Respirology 20(5):819–827
Sutherland K, Chan AS, Ngiam J, Dalci O, Darendeliler MA, Cistulli PA (2018) Awake multimodal phenotyping for prediction of oral appliance treatment outcome. J Clin Sleep Med 14(11):1879–1887
Cartwright RD (1984) Effect of sleep position on sleep apnea severity. Sleep 7(2):110–114
Walter SD, Eliasziw M, Donner A (1998) Sample size and optimal designs for reliability studies. Stat Med 17(1):101–110. https://doi.org/10.1002/(sici)1097-0258(19980115)17:1%3c101::aid-sim727%3e3.0.co;2-e
Chen H, Aarab G, Parsa A, de Lange J, van der Stelt PF, Lobbezoo F (2016) Reliability of three-dimensional measurements of the upper airway on cone beam computed tomography images. Oral Surg Oral Med Oral Pathol Oral Radiol 122(1):104–110
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163
Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Routledge. https://doi.org/10.4324/9780203771587
Ogawa T, Enciso R, Shintaku WH, Clark GT (2007) Evaluation of cross-section airway configuration of obstructive sleep apnea. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 103(1):102–108
Joosten SA, Edwards BA, Wellman A, Turton A, Skuza EM, Berger PJ, Hamilton GS (2015) The effect of body position on physiological factors that contribute to obstructive sleep apnea. Sleep 38(9):1469–1478
McSharry D, O’Connor C, McNicholas T, Langran S, O’Sullivan M, Lowery M, McNicholas WT (2012) Genioglossus fatigue in obstructive sleep apnea. Respir Physiol Neurobiol 183(2):59–66. https://doi.org/10.1016/j.resp.2012.05.024
Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ (2014) Tongue fat and its relationship to obstructive sleep apnea. Sleep 37(10):1639–1648. https://doi.org/10.5665/sleep.4072
Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC (2006) Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol 95(4):2213–2221. https://doi.org/10.1152/jn.00940.2005
Saboisky JP, Butler JE, Gandevia SC, Eckert DJ (2012) Functional role of neural injury in obstructive sleep apnea. Front Neurol 3:95. https://doi.org/10.3389/fneur.2012.00095
Stanchina ML, Malhotra A, Fogel RB, Trinder J, Edwards JK, Schory K, White DP (2003) The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep 26(7):851–856. https://doi.org/10.1093/sleep/26.7.851
Tong J, Jugé L, Burke PG, Knapman F, Eckert DJ, Bilston LE, Amatoury J (2019) Respiratory-related displacement of the trachea in obstructive sleep apnea. J Appl Physiol (Bethesda, Md: 1985) 127(5):1307–1316. https://doi.org/10.1152/japplphysiol.00660.2018
Chen H, Aarab G, de Ruiter MH, de Lange J, Lobbezoo F, van der Stelt PF (2016) Three-dimensional imaging of the upper airway anatomy in obstructive sleep apnea: a systematic review. Sleep Med 21:19–27
Mador MJ, Kufel TJ, Magalang UJ, Rajesh S, Watwe V, Grant BJ (2005) Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest 128(4):2130–2137
Frank M, Ravesloot M, Van Maanen J, Verhagen E, De Lange J, De Vries N (2015) Positional OSA part 1: towards a clinical classification system for position-dependent obstructive sleep apnoea. Sleep Breath 19:473–480
Marklund M, Persson M, Franklin KA (1998) Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest 114(6):1630–1635
Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG (2011) Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med 7(4):376–383. https://doi.org/10.5664/JCSM.1194
Levendowski DJ, Oksenberg A, Vicini C, Penzel T, Levi M, Westbrook PR (2018) A systematic comparison of factors that could impact treatment recommendations for patients with Positional Obstructive Sleep Apnea (POSA). Sleep Med 50:145–151
Funding
Xiaoxin Shi received a scholarship from the China Scholarship Council. This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (Project grant GNT1024351).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study received ethical approval (Sydney Local Health District, Protocol No. X11-0134 & HREC/11/RPAH/192).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
Xiaoxin Shi declares that she has no conflicts of interest. Kate Sutherland has received in kind support from SomnoMed Australia in the form of donation of oral appliances for investigator-initiated research. Frank Lobbezoo is a member of the Academic Advisory Boards for GrindCare and Oral Function of Sunstar Suisse S.A. and receives research grants from Sunstar Suisse S.A., SomnoMed, Vivisol BV, Health Holland, and Airway Management. Erwin Berkhout declares that he has no conflicts of interest. Jan de Lange declares that he has no conflicts of interest. Peter A. Cistulli holds the ResMed Chair in Sleep Medicine at the University of Sydney, established through funding from ResMed. He is a consultant to ResMed, SomnoMed, and Signifier Medical Technologies and has receive equipment support for research. M. Ali Darendeliler declares that he has no conflicts of interest. Oyku Dalci declares that she has no conflicts of interest. Ghizlane Aarab is a member of the Academic Advisory Board for Oral Function of Sunstar Suisse S.A. and receives research grants from Sunstar Suisse S.A., SomnoMed, Vivisol BV, and Health Holland.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, X., Sutherland, K., Lobbezoo, F. et al. Upper airway morphology in adults with positional obstructive sleep apnea. Sleep Breath 28, 193–201 (2024). https://doi.org/10.1007/s11325-023-02879-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-023-02879-0