Abstract
Purpose
To identify a reliable alternative to the full blood [11C]PBR28 quantification method that would be easily replicated in multiple research and clinical settings.
Procedures
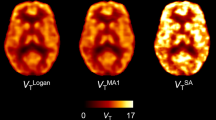
Ten [11C]PBR28 scans were acquired from 7 healthy non-human primates (NHP). Arterial input functions (AIFs) were averaged to create a population template input function (TIF). Population-based input functions were created by scaling the TIF with injected activity per body weight (PBIF) or unmetabolized tracer activity in blood at 15-,30-, and 60-min post-injection (PBIF15, PBIF30, and PBIF60). Two additional input functions were used: the native unmetabolized total plasma activity (Totals) and the Totals curve metabolite corrected by a scaled template parent fraction from a 30-min sample (TPF30-IF). Total distribution volumes (VTs) were calculated using PBIF, PBIF30, PBIF15, PBIF60, Totals, TPF30-IF, and the individual AIF (VTAIF). Distribution volume ratios (DVR) were computed using the cerebellum and the centrum semiovale (CSO), as pseudo-reference regions (DVRCereb, DVRCSO). Results obtained with each method were compared to VTAIF. Applicability of these alternative methods was tested on an independent pharmacological challenge dataset of microglial activation and depletion. Evaluation was carried at baseline, immediately after intervention (acute), and weeks post-intervention (post-recovery).
Results
VTs computed using PBIF15 and PBIF30 showed the best correlation to VTAIF (r > 0.90), while VT derived from the blood-free-scaled PBIF showed poor correlation (r = 0.46) and DVRCSO correlated the least (r = 0.26). In the pharmacological challenge study, most population-derived VT values were comparable to VTAIF at baseline and showed varied sensitivity to challenges at acute and post-recovery evaluation. DVR values did not detect relevant changes.
Conclusions
Population-based input functions scaled with a single blood sample might be a useful alternative to using AIF to compute [11C]PBR28 binding in healthy NHPs or animals with comparable metabolism and overall perform better than pseudo-reference regions approaches.





Similar content being viewed by others
References
Ransohoff RM, Perry VH (2009) Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27:119–145. https://doi.org/10.1146/annurev.immunol.021908.132528
Woods MJ, Williams DC (1996) Multiple forms and locations for the peripheral-type benzodiazepine receptor. Biochem Pharmacol 52:1805–1814. https://doi.org/10.1016/S0006-2952(96)00558-8
Banati RB (2002) Visualising microglial activation in vivo. Glia 40:206–217. https://doi.org/10.1002/glia.10144
Chan GLY, Holden JE, Stoessl AJ, Samii A, Doudet DJ, Dobko T, Morrison KS, Adam M, Schulzer M, Calne DB et al (1999) Reproducibility studies with 11C-DTBZ, a monoamine vesicular transporter inhibitor in healthy human subjects. J Nucl Med 40:283–289
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RSJ (1996) Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab 16:42–52. https://doi.org/10.1097/00004647-199601000-00005
Collste K, Forsberg A, Varrone A, Amini N, Aeinehband S, Yakushev I, Halldin C, Farde L, Cervenka S (2016) Test–retest reproducibility of [11C]PBR28 binding to TSPO in healthy control subjects. Eur J Nucl Med Mol Imaging 43:173–183. https://doi.org/10.1007/s00259-015-3149-8
Takikawa S, Dhawan V, Chaly T, Robeson W, Dahl R, Zanzi I, Mandel F, Eidelberg D, Dahi R, Spetsieris P (1994) input functions for 6-[fluorine-18]fluorodopa quantitation in Parkinsonism: comparative studies and clinical correlations. J Nucl Med 35:955–963
Zanotti-Fregonara P, Maroy R, Peyronneau MA, Trebossen R, Bottlaender M (2012) Minimally invasive input function for 2–18F-fluoro-A-85380 brain PET studies. Eur J Nucl Med Mol Imaging 39:651–659. https://doi.org/10.1007/s00259-011-2004-9
Vriens D, De Geus-Oei LF, Oyen WJG, Visser EP (2009) A curve-fitting approach to estimate the arterial plasma input function for the assessment of glucose metabolic rate and response to treatment. J Nucl Med 50:1933–1939. https://doi.org/10.2967/jnumed.109.065243
Mabrouk R, Strafella AP, Knezevic D, Ghadery C, Mizrahi R, Gharehgazlou A, Koshimori Y, Houle S, Rusjan P (2017) Feasibility study of TSPO quantification with [18F]FEPPA using population-based input function. PLoS ONE 12:1–17. https://doi.org/10.1371/journal.pone.0177785
Schain M, Zanderigo F, Ogden RT, Kreisl WC (2018) Non-invasive estimation of [11C]PBR28 binding potential. Neuroimage 169:278–285. https://doi.org/10.1016/j.neuroimage.2017.12.002
Buchert R, Dirks M, Schütze C, Wilke F, Mamach M, Wirries AK, Pflugrad H, Hamann L, Langer LBN, Wetzel C et al (2020) Reliable quantification of 18F-GE-180 PET neuroinflammation studies using an individually scaled population-based input function or late tissue-to-blood ratio. Eur J Nucl Med Mol Imaging 47:2887–2900. https://doi.org/10.1007/s00259-020-04810-1
Logan J, Fowler JS, Volkow D, Wang G, Ding Y, Alexoff DL (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840
Lyoo CH, Ikawa M, Liow JS, Zoghbi SS, Morse CL, Pike VW, Fujita M, Innis RB, Kreisl WC (2015) Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J Nucl Med 56:701–706. https://doi.org/10.2967/jnumed.114.146027
Zhou X, Ji B, Seki C, Nagai Y, Minamimoto T, Fujinaga M, Zhang MR, Saito T, Saido TC, Suhara T et al (2021) PET imaging of colony-stimulating factor 1 receptor: a head-to-head comparison of a novel radioligand, 11C-GW2580, and 11C-CPPC, in mouse models of acute and chronic neuroinflammation and a rhesus monkey. J Cereb Blood Flow Metab. 1–13 https://doi.org/10.1177/0271678X211004146.
Walker MD, Dinelle K, Kornelsen R, Lee NV, Miao Q, Adam M, Takhar C, Mak E, Schulzer M, Farrer MJ et al (2015) [11C] PBR28 PET imaging is sensitive to neuroinflammation in the aged rat. J Cereb Blood Flow Metab 35:1331–1338. https://doi.org/10.1038/jcbfm.2015.54
Hillmer AT, Holden D, Fowles K, Nabulsi N, West BL, Carson RE, Cosgrove KP (2017) Microglial depletion and activation: a [11C]PBR28 PET study in nonhuman primates. EJNMMI Res 7:4–8. https://doi.org/10.1186/s13550-017-0305-0
Comtat C, Bataille F, Michel C, Jones JP, Sibomana M, Janeiro L, Trebossen R (2004) OSEM-3D reconstruction strategies for the ECAT HRRT. IEEE J Quantum Electron 6:3492–3496. https://doi.org/10.1109/NSSMIC.2004.1466639
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, Macgregor RR, Hitzemann R, Bendriem B, Gatley SJ et al (1990) Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 10:740–747
Renee M, Elmore P, Najafi AR, Koike MA, Nazih N, Spangenberg EE, Rice RA, Kitazawa M, Nguyen H, West BL et al (2015) CSF1 receptor signaling is necessary for microglia viability, which unmasks a cell that rapidly repopulates the microglia-depleted adult brain. Neuron 82:380–397. https://doi.org/10.1016/j.neuron.2014.02.040.CSF1
Zanotti-Fregonara P, Hines CS, Zoghbi SS, Liow JS, Zhang Y, Pike VW, Drevets WC, Mallinger AG, Zarate CA, Fujita M et al (2012) Population-based input function and image-derived input function for [11C](R)-rolipram PET imaging: methodology, validation and application to the study of major depressive disorder. Neuroimage 63:1532–1541. https://doi.org/10.1016/j.neuroimage.2012.08.007
Park E, Gallezot JD, Delgadillo A, Liu S, Planeta B, Lin SF, O’Connor KC, Lim K, Lee JY, Chastre A et al (2015) 11C-PBR28 imaging in multiple sclerosis patients and healthy controls: test-retest reproducibility and focal visualization of active white matter areas. Eur J Nucl Med Mol Imaging 42:1081–1092. https://doi.org/10.1007/s00259-015-3043-4
Zhang W, Guo Y, Wang K, Chen L, Jiang P (2020) Neuroprotective effects of vitamin D and 17ß-estradiol against ovariectomy-induced neuroinflammation and depressive-like state: role of the AMPK/NF-κB pathway. Int Immunopharmacol 86, 106734, https://doi.org/10.1016/j.intimp.2020.106734.
Benedusi V, Meda C, Della Torre S, Monteleone G, Vegeto E, Maggi A (2012) A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology 153:2777–2788. https://doi.org/10.1210/en.2011-1925
Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A (2006) The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology 147:2263–2272. https://doi.org/10.1210/en.2005-1330
Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HEW, Maier SF, Watkins LR (2012) Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 37:1688–1699. https://doi.org/10.1016/j.psyneuen.2012.02.018
Tuisku J, Plavén-Sigray P, Gaiser EC, Airas L, Al-Abdulrasul H, Brück A, Carson RE, Chen MK, Cosgrove KP, Ekblad L et al (2019) Effects of age, BMI and sex on the glial cell marker TSPO — a multicentre [11C]PBR28 HRRT PET study. Eur J Nucl Med Mol Imaging 46:2329–2338. https://doi.org/10.1007/s00259-019-04403-7
Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, Roosa HV, Gage KL, Stathis M, Rais R et al (2014) Regional brain distribution of translocator protein using [11C]DPA-713 PET in individuals infected with HIV. J Neurovirol 20:219–232. https://doi.org/10.1007/s13365-014-0239-5
Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, Rabiner EA, Kalk N, Bishop C, Gunn RN et al (2016) Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology 86:1425–1432. https://doi.org/10.1212/WNL.0000000000002485
Matheson GJ, Plavén-Sigray P, Forsberg A, Varrone A, Farde L, Cervenka S (2017) Assessment of simplified ratio-based approaches for quantification of PET [11C]PBR28 data. EJNMMI Res 7:1–6. https://doi.org/10.1186/s13550-017-0304-1
Hannestad J, Gallezot JD, Schafbauer T, Lim K, Kloczynski T, Morris ED, Carson RE, Ding YS, Cosgrove KP (2012) Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. Neuroimage 63:232–239
Woodcock EA, Schain M, Cosgrove KP, Hillmer AT (2020) Quantification of [11C]PBR28 data after systemic lipopolysaccharide challenge. EJNMMI Res 10:1–6. https://doi.org/10.1186/s13550-020-0605-7
Dimber R, Guo Q, Bishop C, Adonis A, Buckley A, Kocsis A, Owen D, Kalk N, Newbould R, Gunn RN et al (2016) Evidence of brain inflammation in patients with human t-lymphotropic virus type 1-associated myelopathy (HAM): A pilot, multimodal imaging study using 11C-PBR28 PET, MR T1-weighted, and diffusion-weighted imaging. J Nucl Med 57:1905–1912. https://doi.org/10.2967/jnumed.116.175083
Schain M, Zanderigo F, Mann JJ, Ogden RT (2017) Estimation of the binding potential BPND without a reference region or blood samples for brain PET studies. Neuroimage 146:121–131. https://doi.org/10.1016/j.neuroimage.2016.11.035
Canat X, Carayon P, Bouaboula M, Cahard D, Shire D, Roque C, Le Fur G, Casellas P (1993) Distribution profile and properties of peripheral-type benzodiazepine receptors on human hemopoietic cells. Life Sci 52:107–118. https://doi.org/10.1016/0024-3205(93)90293-C
Zanotti-Fregonara P, Liow JS, Fujita M, Dusch E, Zoghbi SS, Luong E, Boellaard R, Pike VW, Comtat C, Innis RB (2011) Image-derived input function for human brain using high resolution PET imaging with [11C](R)-rolipram and [11C]PBR28. PLoS One, 6, https://doi.org/10.1371/journal.pone.0017056.
Zanotti-Fregonara P, Pascual B, Rizzo G, Yu M, Pal N, Beers D, Carter R, Appel SH, Atassi N, Masdeu JC (2018) Head-to-head comparison of 11C-PBR28 and 18F-GE180 for quantification of the translocator protein in the human brain. J Nucl Med 59:1260–1266. https://doi.org/10.2967/jnumed.117.203109
Salmassi A, Lü S, Hedderich J, Oettinghaus C, Jonat W, Mettler L (2001) Interaction of interleukin-6 on human granulosa cell steroid secretion. J Endocrinol 170:471–478. https://doi.org/10.1677/joe.0.1700471
Van der Hoek KH, Woodhouse CM, Brännström M, Norman RJ (1998) Effects of interleukin (IL)-6 on luteinizing hormone- and IL-1β-lnduced ovulation and steroidogenesis in the rat ovary1. Biol Reprod 58:1266–1271. https://doi.org/10.1095/biolreprod58.5.1266
Vinatier D, Dufour P, Tordjeman-Rizzi N, Prolongeau JF, Depret-Moser S, Monnier JC (1995) Immunological aspects of ovarian function: role of the cytokines. Eur J Obstet Gynecol 63:155–168. https://doi.org/10.1016/0301-2115(95)02227-9
Wunder DM, Yared M, Bersinger NA, Widmer D, Kretschmer R, Birkhäuser MH (2006) Serum leptin and C-reactive protein levels in the physiological spontaneous menstrual cycle in reproductive age women. Eur J Endocrinol 155:137–142. https://doi.org/10.1530/eje.1.02178
Wander K, Brindle E, O’Connor KA (2008) C-reactive protein across the menstrual cycle. Am J Phys Anthropol 136:138–146. https://doi.org/10.1002/ajpa.20785
Acknowledgements
We thank the UBC/TRIUMF PET program staff for their contribution to this work: this study would not have been possible without Carolyn English and Siobhan McCormick's assistance for the metabolite analyses radiochemistry laboratories at TRIUMF. Special thanks are due to Julian Kaye and the UBC Animal Care Facilities personnel for their animals' outstanding care. Finally, we thank Dr. Ansel Hillmer for providing the NHP data from Hillmer et al.[17] , and Dr. Richard Carson for an insightful discussion.
Funding
This work was supported by Brain Canada [F14-02712]. [11C]PBR28 was produced at TRIUMF, which is supported by the National Research Council of Canada.
Author information
Authors and Affiliations
Contributions
LAS performed experiments, conducted data analysis, and wrote the manuscript. VS discussed results and revised the manuscript. DJD designed the study, discussed results, and revised the manuscript. All authors edited the manuscript and approved the final submission.
Corresponding author
Ethics declarations
Conflict of Interest
DJD is a Senior Editor for Molecular Imaging and Biology (Neurology). The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aceves-Serrano, L., Sossi, V. & Doudet, D.J. Comparison of Invasive and Non-invasive Estimation of [11C]PBR28 Binding in Non-human Primates. Mol Imaging Biol 24, 404–415 (2022). https://doi.org/10.1007/s11307-021-01661-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01661-6