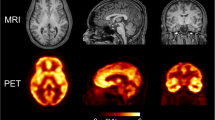
Abstract
Purpose
Quantitative neuroreceptor positron emission tomography (PET) studies often require arterial cannulation to measure input function. While population-based input function (PBIF) would be a less invasive alternative, it has only rarely been used in conjunction with neuroreceptor PET tracers. The aims of this study were (1) to validate the use of PBIF for 2-18F-fluoro-A-85380, a tracer for nicotinic receptors; (2) to compare the accuracy of measures obtained via PBIF to those obtained via blood-scaled image-derived input function (IDIF) from carotid arteries; and (3) to explore the possibility of using venous instead of arterial samples for both PBIF and IDIF.
Methods
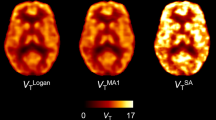
Ten healthy volunteers underwent a dynamic 2-18F-fluoro-A-85380 brain PET scan with arterial and, in seven subjects, concurrent venous serial blood sampling. PBIF was obtained by averaging the normalized metabolite-corrected arterial input function and subsequently scaling each curve with individual blood samples. IDIF was obtained from the carotid arteries using a blood-scaling method. Estimated Logan distribution volume (V T) values were compared to the reference values obtained from arterial cannulation.
Results
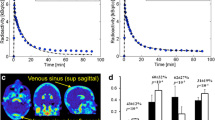
For all subjects, PBIF curves scaled with arterial samples were similar in shape and magnitude to the reference arterial input function. The Logan V T ratio was 1.00 ± 0.05; all subjects had an estimation error <10%. IDIF gave slightly less accurate results (V T ratio 1.03 ± 0.07; eight of ten subjects had an error <10%). PBIF scaled with venous samples yielded inaccurate results (V T ratio 1.13 ± 0.13; only three of seven subjects had an error <10%). Due to arteriovenous differences at early time points, IDIF could not be calculated using venous samples.
Conclusion
PBIF scaled with arterial samples accurately estimates Logan V T for 2-18F-fluoro-A-85380. Results obtained with PBIF were slightly better than those obtained with IDIF. Due to arteriovenous concentration differences, venous samples cannot be substituted for arterial samples.


Similar content being viewed by others
References
Everett BA, Oquendo MA, Abi-Dargham A, Nobler MS, Devanand DP, Lisanby SH, et al. Safety of radial arterial catheterization in PET research subjects. J Nucl Med 2009;50:1742.
Takikawa S, Dhawan V, Spetsieris P, Robeson W, Chaly T, Dahl R, et al. Noninvasive quantitative fluorodeoxyglucose PET studies with an estimated input function derived from a population-based arterial blood curve. Radiology 1993;188:131–6.
Wakita K, Imahori Y, Ido T, Fujii R, Horii H, Shimizu M, et al. Simplification for measuring input function of FDG PET: investigation of 1-point blood sampling method. J Nucl Med 2000;41:1484–90.
Iida H, Itoh H, Bloomfield P, Munaka M, Higano S, Murakami M, et al. A method to quantitate cerebral blood flow using a rotating gamma camera and iodine-123 iodoamphetamine with one blood sampling. Eur J Nucl Med 1994;21:1072–84.
Seike Y, Hashikawa K, Oku N, Moriwaki H, Yamamoto H, Fukuchi K, et al. Evaluation of the use of a standard input function for compartment analysis of [123I]iomazenil data: factors influencing the quantitative results. Ann Nucl Med 2004;18:563–72.
Takikawa S, Dhawan V, Chaly T, Robeson W, Dahl R, Zanzi I, et al. Input functions for 6-[fluorine-18]fluorodopa quantitation in parkinsonism: comparative studies and clinical correlations. J Nucl Med 1994;35:955–63.
Zanotti-Fregonara P, Chen K, Liow JS, Fujita M, Innis RB. Image-derived input function for brain PET studies: many challenges and few opportunities. J Cereb Blood Flow Metab 2011;31:1986–98.
Valette H, Bottlaender M, Dollé F, Guenther I, Fuseau C, Coulon C, et al. Imaging central nicotinic acetylcholine receptors in baboons with [18F]fluoro-A-85380. J Nucl Med 1999;40:1374–80.
Bottlaender M, Valette H, Roumenov D, Dollé F, Coulon C, Ottaviani M, et al. Biodistribution and radiation dosimetry of 18F-fluoro-A-85380 in healthy volunteers. J Nucl Med 2003;44:596–601.
Gallezot JD, Bottlaender M, Grégoire MC, Roumenov D, Deverre JR, Coulon C, et al. In vivo imaging of human cerebral nicotinic acetylcholine receptors with 2-18F-fluoro-A-85380 and PET. J Nucl Med 2005;46:240–7.
Picard F, Bruel D, Servent D, Saba W, Fruchart-Gaillard C, Schöllhorn-Peyronneau MA, et al. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study. Brain 2006;129:2047–60.
Kas A, Bottlaender M, Gallezot JD, Vidailhet M, Villafane G, Grégoire MC, et al. Decrease of nicotinic receptors in the nigrostriatal system in Parkinson’s disease. J Cereb Blood Flow Metab 2009;29:1601–8.
Ellis JR, Villemagne VL, Nathan PJ, Mulligan RS, Gong SJ, Chan JG, et al. Relationship between nicotinic receptors and cognitive function in early Alzheimer’s disease: a 2-[18F]fluoro-A-85380 PET study. Neurobiol Learn Mem 2008;90:404–12.
Mitkovski S, Villemagne VL, Novakovic KE, O’Keefe G, Tochon-Danguy H, Mulligan RS, et al. Simplified quantification of nicotinic receptors with 2[18F]F-A-85380 PET. Nucl Med Biol 2005;32:585–91.
Schöllhorn-Peyronneau M, Coulon C, Bruel D, Ottaviani M, Valette H, Deverre JR, et al. A sensitive and specific quantification of unchanged [18F]-fluoro-A-85380 in plasma by solid phase extraction for human PET studies. Eur J Nucl Med 2005;32:S247.
Meyer PT, Circiumaru V, Cardi CA, Thomas DH, Bal H, Acton PD. Simplified quantification of small animal [18F]FDG PET studies using a standard arterial input function. Eur J Nucl Med Mol Imaging 2006;33:948–54.
Chen K, Bandy D, Reiman E, Huang SC, Lawson M, Feng D, et al. Noninvasive quantification of the cerebral metabolic rate for glucose using positron emission tomography, 18F-fluoro-2-deoxyglucose, the Patlak method, and an image-derived input function. J Cereb Blood Flow Metab 1998;18:716–23.
Chen K, Chen X, Renaut R, Alexander GE, Bandy D, Guo H, et al. Characterization of the image-derived carotid artery input function using independent component analysis for the quantitation of [18F] fluorodeoxyglucose positron emission tomography images. Phys Med Biol 2007;52:7055–71.
Zanotti-Fregonara P, Fadaili el M, Maroy R, Comtat C, Souloumiac A, Jan S, et al. Comparison of eight methods for the estimation of the image-derived input function in dynamic [(18)F]-FDG PET human brain studies. J Cereb Blood Flow Metab 2009;29:1825–35.
Zanotti-Fregonara P, Liow JS, Fujita M, Dusch E, Zoghbi SS, Luong E, et al. Image-derived input function for human brain using high resolution PET imaging with [C](R)-rolipram and [C]PBR28. PLoS One 2011;6:e17056.
Chen K, Ge X, Yao L, Bandy D, Alexander GE, Prouty A, et al. An automated normative-based fluorodeoxyglucose positron emission tomography image-analysis procedure to aid Alzheimer disease diagnosis using statistical parametric mapping and interactive image display. Proc SPIE Int Soc Opt Eng 2006;6144:1638–46.
Caselli RJ, Chen K, Bandy D, Smilovici O, Boeve BF, Osborne D, et al. A preliminary fluorodeoxyglucose positron emission tomography study in healthy adults reporting dream-enactment behavior. Sleep 2006;29:927–33.
Reiman EM, Chen KW, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A 2004;101:284–9.
Zanotti-Fregonara P, Zoghbi SS, Liow JS, Luong E, Boellaard R, Gladding RL, et al. Kinetic analysis in human brain of [11C](R)-rolipram, a positron emission tomographic radioligand to image phosphodiesterase 4: a retest study and use of an image-derived input function. Neuroimage 2011;54:1903–9.
Zanotti-Fregonara P, Maroy R, Comtat C, Jan S, Gaura V, Bar-Hen A, et al. Comparison of 3 methods of automated internal carotid segmentation in human brain PET studies: application to the estimation of arterial input function. J Nucl Med 2009;50:461–7.
Takagi S, Takahashi W, Shinohara Y, Yasuda S, Ide M, Shohtsu A, et al. Quantitative PET cerebral glucose metabolism estimates using a single non-arterialized venous-blood sample. Ann Nucl Med 2004;18:297–302.
Tsuchida T, Sadato N, Yonekura Y, Nakamura S, Takahashi N, Sugimoto K, et al. Noninvasive measurement of cerebral metabolic rate of glucose using standardized input function. J Nucl Med 1999;40:1441–5.
Shiozaki T, Sadato N, Senda M, Ishii K, Tsuchida T, Yonekura Y, et al. Noninvasive estimation of FDG input function for quantification of cerebral metabolic rate of glucose: optimization and multicenter evaluation. J Nucl Med 2000;41:1612–8.
Brock CS, Young H, Osman S, Luthra SK, Jones T, Price PM. Glucose metabolism in brain tumours can be estimated using [18F]2-fluorodeoxyglucose positron emission tomography and a population-derived input function scaled using a single arterialised venous blood sample. Int J Oncol 2005;26:1377–83.
Eberl S, Anayat AR, Fulton RR, Hooper PK, Fulham MJ. Evaluation of two population-based input functions for quantitative neurological FDG PET studies. Eur J Nucl Med 1997;24:299–304.
Ogden RT, Zanderigo F, Choy S, Mann JJ, Parsey RV. Simultaneous estimation of input functions: an empirical study. J Cereb Blood Flow Metab 2010;30:816–26.
Jouvie C, de Gavriloff S, Santiago-Ribeiro MJ, Gaura V, Remy P, Zanotti-Fregonara P, et al. Simultaneous estimation of input functions: the B-SIME method. Biomedical Imaging: From Nano to Macro, 2011 IEEE International Symposium on 30 March–2 April 2011;1758–61.
Guo H, Renaut RA, Chen K. An input function estimation method for FDG-PET human brain studies. Nucl Med Biol 2007;34:483–92.
Mourik JE, Lubberink M, Schuitemaker A, Tolboom N, van Berckel BN, Lammertsma AA, et al. Image-derived input functions for PET brain studies. Eur J Nucl Med Mol Imaging 2009;36:463–71.
Chiou WL. The phenomenon and rationale of marked dependence of drug concentration on blood sampling site. Implications in pharmacokinetics, pharmacodynamics, toxicology and therapeutics (part II). Clin Pharmacokinet 1989;17:275–90.
Chiou WL. The phenomenon and rationale of marked dependence of drug concentration on blood sampling site. Implications in pharmacokinetics, pharmacodynamics, toxicology and therapeutics (part I). Clin Pharmacokinet 1989;17:175–99.
Greuter H, Lubberink M, Hendrikse NH, Van der Veldt AA, Wong Y, Schuit R, et al. Venous versus arterial blood samples for plasma input pharmacokinetic analysis of different radiotracer PET studies. SNM conference proceedings 2011;52:(Suppl 1):1974.
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 1979;6:371–88.
Moriwaki H, Matsumoto M, Hashikawa K, Oku N, Okazaki Y, Handa N, et al. Quantitative assessment of cerebral blood flow by 123I-IMP SPECT: venous sampling method with hand warming in the water bath. Kaku Igaku 1993;30:481–8.
Backes H, Ullrich R, Neumaier B, Kracht L, Wienhard K, Jacobs AH. Noninvasive quantification of (18)F-FLT human brain PET for the assessment of tumour proliferation in patients with high-grade glioma. Eur J Nucl Med Mol Imaging 2009;36:1960–7.
Wahl LM, Asselin MC, Nahmias C. Regions of interest in the venous sinuses as input functions for quantitative PET. J Nucl Med 1999;40:1666–75.
Wong DF, Young D, Wilson PD, Meltzer CC, Gjedde A. Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors: theory, validation, and changes during normal aging. J Cereb Blood Flow Metab 1997;17:316–30.
Acknowledgments
The authors are grateful to Ioline Henter for invaluable editorial assistance.
Financial support
This study was supported in part by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zanotti-Fregonara, P., Maroy, R., Peyronneau, MA. et al. Minimally invasive input function for 2-18F-fluoro-A-85380 brain PET studies. Eur J Nucl Med Mol Imaging 39, 651–659 (2012). https://doi.org/10.1007/s00259-011-2004-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-2004-9