Abstract
Purpose
The main objective of the present study was to compare the 2-deoxy-2-[18F]fluoro-D-glucose ([18F]-FDG) and 3′-[18F]fluoro-3′-deoxythymidine ([18F]-FLT) PET imaging biomarkers for the longitudinal follow-up of small animal proton therapy studies in the context of hepatocellular carcinoma (HCC).
Procedures
SK-HEP-1 cells were injected into NMRI nude mice to mimic human HCC. The behavior of [18F]-FDG and [18F]-FLT tumor uptake was evaluated after proton therapy procedures. The proton single-fraction doses were 5, 10, and 20 Gy, with a dose rate of 10 Gy/min. The experimental protocol consisted of 8 groups of 10 mice, each group experiencing a particular dose/radiotracer condition. A reference PET exam was performed on each mouse the day before the irradiation procedure, followed by PET exams every 3 days up to 16 days after irradiation.
Results
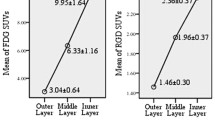
[18F]-FDG uptake showed a linear dose-dependent increase in the first days after treatment (37%, p < 0.05), while [18F]-FLT uptake decreased in a dose-dependent manner (e.g., 21% for 5 Gy compared to 10 Gy, p = 1.1e-2). At the later time point, [18F]-FDG normalized activity showed an 85% decrease (p < 0.01) for both 10 and 20 Gy doses and no variation for 5 Gy. Conversely, a significant 61% (p = 0.002) increase was observed for [18F]-FLT normalized activity at 5 Gy and no variation for higher doses.
Conclusion
We showed that the use of the [18F]-FDG and [18F]-FLT radiolabeled molecules can provide useful and complementary information for longitudinal follow-up of small animal proton therapy studies in the context of HCC. [18F]-FDG PET imaging enables a treatment monitoring several days/weeks postirradiation. On the other hand, [18F]-FLT could represent a good candidate to monitor the treatment few days postirradiation, in the context of hypo-fractioned and close irradiation planning. This opens new perspectives in terms of treatment efficacy verification depending on the irradiation scheme.






Similar content being viewed by others
References
Ohri N, Dawson LA, Krishnan S, Seong J, Cheng JC, Sarin SK, Kinkhabwala M, Ahmed MM, Vikram B, Coleman CN, Guha C (2016) Radiotherapy for hepatocellular carcinoma: new indications and directions for future study. J Natl Cancer Inst 108:djw133
Yoo GS, Yu JI, Park HC (2018) Proton therapy for hepatocellular carcinoma: current knowledges and future perspectives. World J Gastroenterol 24:3090–3100
Klein J, Dawson LA (2013) Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 87:22–32
Dionisi F, Widesott L, Lorentini S, Amichetti M (2014) Is there a role for proton therapy in the treatment of hepatocellular carcinoma? A systematic review. Radiother Oncol 111:1–10
Eggert T, Greten TF (2017) Current standard and future perspectives in non-surgical therapy for hepatocellular carcinoma. Digestion 96:1–4
Yeung RH, Chapman TR, Bowen SR, Apisarnthanarax S (2017) Proton beam therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 17:911–924
Grégoire V, Thorwarth D, Lee JA (2018) Molecular imaging-guided radiotherapy for the treatment of head-and-neck squamous cell carcinoma: does it fulfill the promises? Semin Radiat Oncol 28:35–45
Bajpai S, Kambadakone A, Guimaraes AR, Arellano RS, Gervais DA, Sahani D (2015) Image-guided treatment in the hepatobiliary system: role of imaging in treatment planning and posttreatment evaluation. Radiographics 35:1393–1418
Huang Y-C, Lu H-I, Huang S-C, Hsu CC, Chiu NT, Wang YM, Chiu YC, Li SH (2017) FDG PET using SUVmax for preoperative T-staging of esophageal squamous cell carcinoma with and without neoadjuvant chemoradiotherapy. BMC Med Imaging 17:1
Cremonesi M, Gilardi L, Ferrari ME, Piperno G, Travaini LL, Timmerman R, Botta F, Baroni G, Grana CM, Ronchi S, Ciardo D, Jereczek-Fossa BA, Garibaldi C, Orecchia R (2017) Role of interim 18F-FDG-PET/CT for the early prediction of clinical outcomes of non-small cell lung cancer (NSCLC) during radiotherapy or chemo-radiotherapy. A systematic review. Eur J Nucl Med Mol Imaging 44:1915–1927
Rafat M, Ali R, Graves EE (2015) Imaging radiation response in tumor and normal tissue. Am J Nucl Med Mol Imaging 5:317–332
Cliffe H, Patel C, Prestwich R, Scarsbrook A (2017) Radiotherapy response evaluation using FDG PET-CT-established and emerging applications. Br J Radiol 90:20160764
Decazes P, Thureau S, Dubray B, Vera P (2018) How to use PET/CT in the evaluation of response to radiotherapy. Q J Nucl Med Mol Imaging 62:152–164
Ryttlefors M, Danfors T, Latini F, Montelius A, Blomquist E, Gudjonsson O (2016) Long-term evaluation of the effect of hypofractionated high-energy proton treatment of benign meningiomas by means of (11)C-L-methionine positron emission tomography. Eur J Nucl Med Mol Imaging 43:1432–1443
van Elmpt W, Ollers M, Dingemans A-MC, Lambin P, de Ruysscher D (2012) Response assessment using 18F-FDG PET early in the course of radiotherapy correlates with survival in advanced-stage non-small cell lung cancer. J Nucl Med 53:1514–1520
Pommier P, Touboul E, Chabaud S, Dussart S, le Pechoux C, Giammarile F, Carrie C (2010) Impact of (18)F-FDG PET on treatment strategy and 3D radiotherapy planning in non-small cell lung cancer: a prospective multicenter study. AJR Am J Roentgenol 195:350–355
Hoeben BAW, Troost EGC, Span PN, van Herpen CML, Bussink J, Oyen WJG, Kaanders JHAM (2013) 18F-FLT PET during radiotherapy or chemoradiotherapy in head and neck squamous cell carcinoma is an early predictor of outcome. J Nucl Med 54:532–540
Eckel F, Herrmann K, Schmidt S, Hillerer C, Wieder HA, Krause BJ, Schuster T, Langer R, Wester HJ, Schmid RM, Schwaiger M, Buck AK (2009) Imaging of proliferation in hepatocellular carcinoma with the in vivo marker 18F-fluorothymidine. J Nucl Med 50:1441–1447
Lin C, Kume K, Mori T, Martinez ME, Okazawa H, Kiyono Y (2015) Predictive value of early-stage uptake of 3’-deoxy-3’-18F-fluorothymidine in cancer cells treated with charged particle irradiation. J Nucl Med 56:945–950
Ford E, Emery R, Huff D, Narayanan M, Schwartz J, Cao N, Meyer J, Rengan R, Zeng J, Sandison G, Laramore G, Mayr N (2017) An image-guided precision proton radiation platform for preclinical in vivo research. Phys Med Biol 62:43–58
Almeida IP, Vaniqui A, Schyns LE et al (2019) Exploring the feasibility of a clinical proton beam with an adaptive aperture for pre-clinical research. Br J Radiol 92:20180446
Greubel C, Assmann W, Burgdorf C, Dollinger G, du G, Hable V, Hapfelmeier A, Hertenberger R, Kneschaurek P, Michalski D, Molls M, Reinhardt S, Röper B, Schell S, Schmid TE, Siebenwirth C, Wenzl T, Zlobinskaya O, Wilkens JJ (2011) Scanning irradiation device for mice in vivo with pulsed and continuous proton beams. Radiat Environ Biophys 50:339–344
Meyer J, Stewart RD, Smith D, Eagle J, Lee E, Cao N, Ford E, Hashemian R, Schuemann J, Saini J, Marsh S, Emery R, Dorman E, Schwartz J, Sandison G (2017) Biological and dosimetric characterisation of spatially fractionated proton minibeams. Phys Med Biol 62:9260–9281
Vanstalle M, Constanzo J, Finck C (2019) Investigation of optimal physical parameters for precise proton irradiation of orthotopic tumors in small animals. Int J Radiat Oncol Biol Phys 103:1241–1250
Tai Y, Gao J-H, Zhao C, Tong H, Zheng SP, Huang ZY, Liu R, Tang CW, Li J (2018) SK-Hep1: not hepatocellular carcinoma cells but a cell model for liver sinusoidal endothelial cells. Int J Clin Exp Pathol 11:2931–2938
Eun JR, Jung YJ, Zhang Y, Zhang Y, Tschudy-Seney B, Ramsamooj R, Wan YJY, Theise ND, Zern MA, Duan Y (2014) Hepatoma SK Hep-1 cells exhibit characteristics of oncogenic mesenchymal stem cells with highly metastatic capacity. PLoS One 9:e110744
Altmeyer A, Jung AC, Ignat M, Benzina S, Denis JM, Gueulette J, Noël G, Mutter D, Bischoff P (2010) Pharmacological enhancement of autophagy induced in a hepatocellular carcinoma cell line by high-LET radiation. Anticancer Res 30:303–310
Marchand P, Ouadi A, Pellicioli M, Schuler J, Laquerriere P, Boisson F, Brasse D (2016) Automated and efficient radiosynthesis of [(18)F]FLT using a low amount of precursor. Nucl Med Biol 43:520–527
Vanstalle M, Constanzo J, Karakaya Y, Finck C, Rousseau M, Brasse D (2018) Analytical dose modeling for preclinical proton irradiation of millimetric targets. Med Phys 45:470–478
Constanzo J, Vanstalle M, Finck C, Brasse D, Rousseau M (2019) Dosimetry and characterization of a 25-MeV proton beam line for preclinical radiobiology research. Med Phys 46:2356–2362
Wilson GD, Thibodeau BJ, Fortier LE, Pruetz BL, Galoforo S, Baschnagel AM, Chunta J, Oliver Wong CY, Yan D, Marples B, Huang J (2014) Glucose metabolism gene expression patterns and tumor uptake of 18F-fluorodeoxyglucose after radiation treatment. Int J Radiat Oncol Biol Phys 90:620–627
Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, Phelps ME, Weber WA (2006) Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med 47:999–1006
Loening AM, Gambhir SS (2003) AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2:131–137
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
McKinney W (2010) Data structures for statistical computing in python. Proceedings of the 9th Python in Science Conference 56–61
Virtanen P, Gommers R, Oliphant TE et al (2020) SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 17:261–272
Kuntner C, Stout DB (2014) Quantitative preclinical PET imaging: opportunities and challenges. Front Phys 2:1-12
Schaub SK, Hartvigson PE, Lock MI, Høyer M, Brunner TB, Cardenes HR, Dawson LA, Kim EY, Mayr NA, Lo SS, Apisarnthanarax S (2018) Stereotactic body radiation therapy for hepatocellular carcinoma: current trends and controversies. Technol Cancer Res Treat 17:153303381879021
Higashi K, Clavo AC, Wahl RL (1993) In vitro assessment of 2-fluoro-2-deoxy-D-glucose, L-methionine and thymidine as agents to monitor the early response of a human adenocarcinoma cell line to radiotherapy. J Nucl Med 34:773–779
Chalkidou A, Landau DB, Odell EW, Cornelius VR, O’Doherty MJ, Marsden PK (2012) Correlation between Ki-67 immunohistochemistry and 18F-fluorothymidine uptake in patients with cancer: a systematic review and meta-analysis. Eur J Cancer 48:3499–3513
Tehrani OS, Shields AF (2013) PET imaging of proliferation with pyrimidines. J Nucl Med 54:903–912
Sharma R, Inglese M, Dubash S, Lu H, Pinato DJ, Sanghera C, Patel N, Chung A, Tait PD, Mauri F, Crum WR, Barwick TD, Aboagye EO (2020) Monitoring response to transarterial chemoembolization in hepatocellular carcinoma using 18F-fluorothymidine PET. J Nucl Med 61:1743–1748
Ronot M, Clift AK, Vilgrain V, Frilling A (2016) Functional imaging in liver tumours. J Hepatol 65:1017–1030
Lu R-C, She B, Gao W-T, Ji YH, Xu DD, Wang QS, Wang SB (2019) Positron-emission tomography for hepatocellular carcinoma: current status and future prospects. World J Gastroenterol 25:4682–4695
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236
Acknowledgements
This work was partly supported by ITMO Cancer AVIESAN (Alliance Nationale pour les Sciences de la Vie et de la Santé, National Alliance for Life Sciences and Health) within the framework of the Cancer Plan and by IN2P3. The authors wish to thank Bruno Jessel, Lionel Thomas, Jacky Schuler, Michel Pellicioli, Julie Constanzo, and Cédric Mathieu for their help in performing all the experiments. We also thank all the members of the mechanical workshop involved in the development of the beam line.
Author information
Authors and Affiliations
Contributions
All authors discussed and conceived the generic concept and reviewed the manuscript. D.B. wrote the main part of the manuscript. P.M. and A.O. contributed to the PET experiments. M.R., M.V. and C.F. participated in the setup of the preclinical proton beam line and the associated dosimetry. H.B. performed the cell culture and the immunohistochemical procedure and analyses. P.L. performed the statistical analyses. F.B and D.B analyzed the PET data.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brasse, D., Burckel, H., Marchand, P. et al. Comparison of the [18F]-FDG and [18F]-FLT PET Tracers in the Evaluation of the Preclinical Proton Therapy Response in Hepatocellular Carcinoma. Mol Imaging Biol 23, 724–732 (2021). https://doi.org/10.1007/s11307-021-01602-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01602-3