Abstract
Purpose
Creatine (Cr) is a major metabolite in the bioenergetic system. Measurement of Cr using conventional MR spectroscopy (MRS) suffers from low spatial resolution and relatively long acquisition times. Creatine chemical exchange saturation transfer (CrCEST) magnetic resonance imaging (MRI) is an emerging molecular imaging method for tissue Cr measurements. Our previous study showed that the CrCEST contrast, obtained through multicomponent Z-spectral fitting, was lower in tumors compared to normal brain, which further reduced with tumor progression. The current study was aimed to investigate if CrCEST MRI can also be useful for differentiating gliomas with different degrees of aggressiveness.
Procedures
Intracranial 9L gliosarcoma and F98 glioma bearing rats with matched tumor size were scanned with a 9.4 T MRI scanner at two time points. CEST Z-spectra were collected using a customized sequence with a frequency-selective rectangular saturation pulse (B1 = 50 Hz, duration = 3 s) followed by a single-shot readout. Z spectral data were fitted pixel-wise with five Lorentzian functions, and maps of CrCEST peak amplitude, linewidth, and integral were produced. For comparison, single-voxel proton MR spectroscopy (1H-MRS) was performed to quantify and compare the total Cr concentration in the tumor.
Results
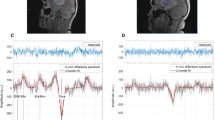
CrCEST contrasts decreased with tumor progression from weeks 3 to 4 in both 9L and F98 phenotypes. More importantly, F98 tumors had significantly lower CrCEST integral compared to 9L tumors. On the other hand, integrals of other Z-spectral components were unable to differentiate both tumor progression and phenotype with limited sample size.
Conclusions
Given that F98 is a more aggressive tumor than 9L, this study suggests that CrCEST MRI may help differentiate gliomas with different aggressiveness.





Similar content being viewed by others
References
Bleyer WA (1999) Epidemiologic impact of children with brain tumors. Childs Nerv Syst 15:758–763
Panigrahy A, Bluml S (2009) Neuroimaging of pediatric brain tumors: from basic to advanced magnetic resonance imaging (MRI). J Child Neurol 24:1343–1365
Allison GT, Fujiwara T (2002) The relationship between EMG median frequency and low frequency band amplitude changes at different levels of muscle capacity. Clin Biomech 17:464–469
Lin A, Bluml S, Mamelak AN (1999) Efficacy of proton magnetic resonance spectroscopy in clinical decision making for patients with suspected malignant brain tumors. J Neurooncol 45:69–81
Wong ET, Jackson EF, Hess KR et al (1998) Correlation between dynamic MRI and outcome in patients with malignant gliomas. Neurology 50:777–781
Dean BL, Drayer BP, Bird CR et al (1990) Gliomas: classification with MR imaging. Radiology 174:411–415
Watanabe M, Tanaka R, Takeda N (1992) Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 34:463–469
Kondziolka D, Lunsford LD, Martinez AJ (1993) Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg 79:533–536
Ginsberg LE, Fuller GN, Hashmi M et al (1998) The significance of lack of MR contrast enhancement of supratentorial brain tumors in adults: histopathological evaluation of a series. Surg Neurol 49:436–440
Forsen S, Hoffman R (1963) Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys 39:2892–2901
Wolff S, Balaban R (1990) NMR imaging of labile proton-exchange. J Magn Reson 86:164–169
Ward KM, Aletras AH, Balaban RS (2000) A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 143:79–87
Sherry AD, Woods M (2008) Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Ann Rev Biomed Eng 10:391–411
Zhou J, van Zijl PC (2006) Chemical exchange saturation transfer imaging and spectroscopy. Prog Nucl Magn Reson Spectrosc 48:109–136
van Zijl PC, Yadav NN (2011) Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med 65:927–948
Kogan F, Hariharan H, Reddy R (2013) Chemical Exchange Saturation Transfer (CEST) imaging: description of technique and potential clinical applications. Curr Radiol Rep 1:102–114
Cai K, Haris M, Singh A et al (2012) Magnetic resonance imaging of glutamate. Nat Med 18:302–306
Haris M, Nanga RP, Singh A et al (2012) Exchange rates of creatine kinase metabolites: feasibility of imaging creatine by chemical exchange saturation transfer MRI. NMR Biomed 25:1305–1309
Zhou J, Lal B, Wilson DA et al (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50:1120–1126
van Zijl PC, Jones CK, Ren J et al (2007) MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST). Proc Natl Acad Sci U S A 104:4359–4364
Ling W, Regatte RR, Navon G et al (2008) Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc Natl Acad Sci U S A 105:2266–2270
Walker-Samuel S, Ramasawmy R, Torrealdea F et al (2013) In vivo imaging of glucose uptake and metabolism in tumors. Nat Med 19:1067–1072
Cai K, Singh A, Roalf DR et al (2013) Mapping glutamate in subcortical brain structures using high-resolution GluCEST MRI. NMR Biomed 26:1278–1284
Haris M, Nath K, Cai K et al (2013) Imaging of glutamate neurotransmitter alterations in Alzheimer’s disease. NMR Biomed 26:386–391
Haris M, Singh A, Cai K et al (2014) High resolution mapping of modafinil induced changes in glutamate level in rat brain. PLoS One 9:e103154
Haris M, Cai K, Singh A et al (2011) In vivo mapping of brain myo-inositol. Neuroimage 54:2079–2085
Haris M, Singh A, Cai K et al (2013) MICEST: a potential tool for non-invasive detection of molecular changes in Alzheimer’s disease. J Neurosci Methods 12:87–93
Cai K, Singh A, Poptani H et al (2015) CEST signal at 2ppm (CEST@2ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed 28:1–8
Haris M, Singh A, Cai K et al (2014) A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med 20:209–214
Kogan F, Haris M, Singh A et al (2014) Method for high-resolution imaging of creatine in vivo using chemical exchange saturation transfer. Magn Reson Med 71:164–172
Kogan F, Haris M, Debrosse C et al (2014) In vivo chemical exchange saturation transfer imaging of creatine (CrCEST) in skeletal muscle at 3T. J Magn Reson Imaging 40:596–602
Kuszewski JJ, Thottungal RA, Clore GM et al (2008) Automated error-tolerant macromolecular structure determination from multidimensional nuclear Overhauser enhancement spectra and chemical shift assignments: improved robustness and performance of the PASD algorithm. J Biomol NMR 41:221–239
Stadlbauer A, Gruber S, Nimsky C et al (2006) Preoperative grading of gliomas by using metabolite quantification with high-spatial-resolution proton MR spectroscopic imaging. Radiology 238:958–969
Belitzer V, Tsybakova E (1939) About mechanism of phosphorylation, respiratory coupling. Biochimie 4:516–533
Gill SS, Thomas DG, Van Bruggen N et al (1990) Proton MR spectroscopy of intracranial tumours: in vivo and in vitro studies. J Comput Assist Tomogr 14:497–504
Fenstermacher MJ, Narayana PA (1990) Serial proton magnetic resonance spectroscopy of ischemic brain injury in humans. Invest Radiol 25:1034–1039
Usenius JP, Vainio P, Hernesniemi J et al (1994) Choline-containing compounds in human astrocytomas studied by 1H NMR spectroscopy in vivo and in vitro. J Neurochem 63:1538–1543
Chang L, McBride D, Miller BL et al (1995) Localized in vivo 1H magnetic resonance spectroscopy and in vitro analyses of heterogeneous brain tumors. J Neuroimaging 5:157–163
Peeling J, Sutherland G (1992) High-resolution 1H NMR spectroscopy studies of extracts of human cerebral neoplasms. Magn Reson Med 24:123–136
Doblas S, He T, Saunders D et al (2010) Glioma morphology and tumor-induced vascular alterations revealed in seven rodent glioma models by in vivo magnetic resonance imaging and angiography. J Magn Reson Imaging 32:267–275
Vonarbourg A, Sapin A, Lemaire L et al (2004) Characterization and detection of experimental rat gliomas using magnetic resonance imaging. MAGMA 17:133–139
Barth RF, Kaur B (2009) Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neuro-Oncol 94:299–312
Recinos VR, Tyler BM, Bekelis K et al (2010) Combination of intracranial temozolomide with intracranial carmustine improves survival when compared with either treatment alone in a rodent glioma model. Neurosurgery 66:530–537, discussion 537
Kim S, Pickup S, Hsu O et al (2008) Diffusion tensor MRI in rat models of invasive and well-demarcated brain tumors. NMR Biomed 21:208–216
Tkac I, Starcuk Z, Choi IY et al (1999) In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 41:649–656
O’Gorman RL, Michels L, Edden RA et al (2011) In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J Magn Reson Imaging 33:1262–1267
Naressi A, Couturier C, Castang I et al (2001) Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 31:269–286
Naressi A, Couturier C, Devos JM et al (2001) Java-based graphical user interface for the MRUI quantitation package. MAGMA 12:141–152
Ramani A, Dalton C, Miller DH et al (2002) Precise estimate of fundamental in-vivo MT parameters in human brain in clinically feasible times. Magn Reson Imaging 20:721–731
Jin T, Wang P, Zong X et al (2013) MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Imaging 69:760–770
Zhou J (2011) Amide proton transfer imaging of the human brain. Methods Mol Biol 711:227–237
Singh A, Cai K, Haris M et al (2013) On B1 inhomogeneity correction of in vivo human brain glutamate chemical exchange saturation transfer contrast at 7T. Magn Reson Med 69:818–824
Cai K, Xu HN, Singh A et al (2014) Breast cancer redox heterogeneity detectable with chemical exchange saturation transfer (CEST) MRI. Mol Imaging Biol 16:670–679
Cai K, Xu HN, Singh A et al (2013) Characterizing prostate tumor mouse xenografts with CEST and MT-MRI and redox scanning. Adv Exp Med Biol 765:39–45
Walker EA, Fenton ME, Salesky JS et al (2011) Magnetic resonance imaging of benign soft tissue neoplasms in adults. Radiol Clin N Am 49(1197–1217):vi
Zaiss M, Xu J, Goerke S et al (2014) Inverse Z-spectrum analysis for spillover-, MT-, and T1-corrected steady-state pulsed CEST-MRI—application to pH-weighted MRI of acute stroke. NMR Biomed 27:240–252
Patra S, Ghosh A, Roy SS et al (2012) A short review on creatine-creatine kinase system in relation to cancer and some experimental results on creatine as adjuvant in cancer therapy. Amino Acids 42:2319–2330
Pretlow TG, Whitehurst GB, Pretlow TP et al (1982) Decrease in creatine kinase in human prostatic carcinoma compared to benign prostatic hyperplasia. Cancer Res 42:4842–4848
Joseph J, Cardesa A, Carreras J (1997) Creatine kinase activity and isoenzymes in lung, colon and liver carcinomas. Br J Cancer 76:600–605
Tsung SH (1983) Creatine kinase activity and isoenzyme pattern in various normal tissues and neoplasms. Clin Chem 29:2040–2043
Onda T, Uzawa K, Endo Y et al (2006) Ubiquitous mitochondrial creatine kinase downregulated in oral squamous cell carcinoma. Br J Cancer 94:698–709
Patra S, Bera S, SinhaRoy S et al (2008) Progressive decrease of phosphocreatine, creatine and creatine kinase in skeletal muscle upon transformation to sarcoma. FEBS J 275:3236–3247
Fukuda J, Yagishita S, Yamaoka K et al (1994) Creatine kinase isoenzyme BB increased in serum and tumor tissue of patients with giant cell tumor of bone. Clin Chem 40:2064–2065
Meffert G, Gellerich FN, Margreiter R et al (2005) Elevated creatine kinase activity in primary hepatocellular carcinoma. Boston Med Ctr Gastroenterol 5:9
Tain RLW, Yang S, Zhou XJ et al (2015) Separated quantification of creatine and phosphocreatine based on a novel proton MR method combing 1H-MRS and CEST MRI. Process Intl Soc Magn Reson Med 23:3352
Mori S, Eleff SM, Pilatus U et al (1998) Proton NMR spectroscopy of solvent-saturable resonances: a new approach to study pH effects in situ. Magn Reson Med 40:36–42
Jin T, Wang P, Zong X et al (2012) Magnetic resonance imaging of the Amine-Proton EXchange (APEX) dependent contrast. Neuroimage 59:1218–1227
Desmond KL, Moosvi F, Stanisz GJ (2014) Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7 T. Magn Reson Med 71:1841–1853
Sun PZ, Benner T, Kumar A et al (2008) Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn Reson Med 60:834–841
Arus C, Barany M, Westler WM et al (1984) 1H NMR of intact muscle at 11 T. FEBS Lett 165:231–237
Middleton DA, Hockings PD, Glen S et al (1995) Image directed proton spectroscopy of gerbil brain at 7 tesla. NMR Biomed 8:118–126
Wu LM, Zhou B, Lu Q et al (2016) T2* relaxation time in the detection and assessment of aggressiveness of peripheral zone cancer in comparison with diffusion-weighted imaging. Clin Radiol 71:356–362
Simpkin CJ, Morgan VA, Giles SL et al (2013) Relationship between T2 relaxation and apparent diffusion coefficient in malignant and non-malignant prostate regions and the effect of peripheral zone fractional volume. Br J Radiol 86:20120469
Just M, Thelen M (1988) Tissue characterization with T1, T2, and proton density values: results in 160 patients with brain tumors. Radiology 169:779–785
Felix R, Schorner W, Laniado M et al (1985) Brain tumors: MR imaging with gadolinium-DTPA. Radiology 156:681–688
Aronen HJ, Gazit IE, Louis DN et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Shin JH, Lee HK, Kwun BD et al (2002) Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas: preliminary results. Am J Roentgenol 179:783–789
Arnold DL, Shoubridge EA, Villemure JG et al (1990) Proton and phosphorus magnetic resonance spectroscopy of human astrocytomas in vivo. Preliminary observations on tumor grading. NMR Biomed 3:184–189
Bruhn H, Frahm J, Gyngell ML et al (1989) Noninvasive differentiation of tumors with use of localized H-1 MR spectroscopy in vivo: initial experience in patients with cerebral tumors. Radiology 172:541–548
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. Am J Neuroradiol 24:1989–1998
Acknowledgments
We gratefully acknowledge the discussions with Drs. Mohammad Haris and Anup Singh from the Center for MR and Optical Imaging at University of Pennsylvania. Our sincere thanks are also due to Drs. Weixia Liu and Stephen Pickup for their imaging assistance as well as Ranjit Ittyerah and Dr. Damodar Reddy for their help with the animal model. This work was partially supported by the National Center for Research Resources and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health through grant number P41 EB015893 and the Department of Radiology and the Center for Magnetic Resonance Research at University of Illinois at Chicago.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cai, K., Tain, RW., Zhou, X.J. et al. Creatine CEST MRI for Differentiating Gliomas with Different Degrees of Aggressiveness. Mol Imaging Biol 19, 225–232 (2017). https://doi.org/10.1007/s11307-016-0995-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0995-0