Abstract
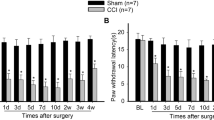
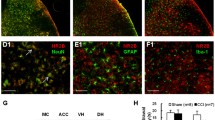
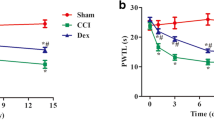
Neuropathic pain is a refractory pain state, and its mechanism is still not clear. Previous studies have shown that the purine receptor P2X4R expressed on hyperactive microglia in the spinal cord is essential for the occurrence and development of neuropathic pain. The cerebrospinal fluid-contacting nucleus (CSF-contacting nucleus) in the midbrain has been found to play an important role in the descending inhibition system of modulation. However, there have been no studies on P2X4R in the CSF-contacting nucleus involved in neuropathic pain. To investigate whether P2X4R is expressed in the CSF-contacting nucleus and whether its expression in the CSF-contacting nucleus is involved in the regulation of neuropathic pain, we used a model of chronic sciatic nerve ligation injury (CCI) to simulate neuropathic pain conditions. Immunohistochemistry experiments were conducted to identify the expression of P2X4R in the CSF-contacting nuclei in CCI rats, and western blot analysis showed a significant increase in P2X4R levels 7 days after modeling. Then, we packaged a P2rx4 gene-targeting shRNA in scAAV9 to knock down the P2X4R level in the CSF-contacting nucleus, and we found that CCI-induced mechanical hyperalgesia was reversed. In conclusion, P2X4R expressed in the CSF-contacting nucleus is involved in the process of neuropathic pain, and downregulating P2X4R protein in the CSF-contacting nucleus can reverse the occurrence and development of hyperalgesia, which could represent a potent therapeutic strategy for neuropathic pain.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aby F, Whitestone S, Landry M, Ulmann L, Fossat P (2018) Inflammatory-induced spinal dorsal horn neurons hyperexcitability is mediated by P2X4 receptors. Pain rep 3:e660. https://doi.org/10.1097/pr9.0000000000000660
Bennett G, Xie Y (1988) A peripheral mononeuropathy in rat that produces disorder of pain sendation like those seen in man. Pain 33:87–107. https://doi.org/10.1016/0304-3959(88)90209-6
Bernier LP, Ase AR, Seguela P (2018) P2X receptor channels in chronic pain pathways. Br J Pharmacol 175:2219–2230. https://doi.org/10.1111/bph.13957
Chao CL, Lu XF, Zhang LC (2010) Formalin-induced pain stimulation induced expression of GABA in the distal cerebrospinal fluid contacting neurons. Zhongguo Ying Yong Sheng Li Xue Za Zhi 26:36–38
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. https://doi.org/10.1016/0165-0270(94)90144-9
Dixon W (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462. https://doi.org/10.1146/annurev.pa.20.040180.002301
Fei Y, Wang X, Chen S, Zhou Q, Zhang C, Li Y, Sun L, Zhang L (2016) Role of the RVM in descending pain regulation originating from the cerebrospinal fluid-contacting nucleus. Neurochem Res 41:1651–1661. https://doi.org/10.1007/s11064-016-1880-6
Gilron I, Baron R, Jensen T (2015) Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc 90:532–545. https://doi.org/10.1016/j.mayocp.2015.01.018
Gonzales E, Prigent S, Abou-Lovergne A, Boucherie S, Tordjmann T, Jacquemin E, Combettes L (2007) rat hepatocytes express functional P2X receptors. FEBS Lett 581:3260–3266. https://doi.org/10.1016/j.febslet.2007.06.016
Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, Treede RD (2011) A new definition of neuropathic pain. Pain 152:2204–2205. https://doi.org/10.1016/j.pain.2011.06.017
Jurga AM, Piotrowska A, Makuch W, Przewlocka B, Mika J (2017) Blockade of P2X4 receptors inhibits neuropathic pain-related behavior by preventing MMP-9 activation and, consequently, pronociceptive interleukin release in a rat model. Front Pharmacol 8:48 https://doi.org/10.3389/fphar.2017.00048
Li L, Cao J, Zhang S, Wang C, Wang J, Song G, Wang H, Zhang L (2014) NCAM signaling mediates the effects of GDNF on chronic morphine-induced neuroadaptations. J Molecular Neurosci : MN 53:580–589. https://doi.org/10.1007/s12031-013-0224-0
Liu H, Yan WW, Lu XX, Zhang XL, Wei JQ, Wang XY, Wang T, Wu T, Cao J, Shao CJ, Zhou F, Zhang HX, Zhang P, Zang T, Lu XF, Cao JL, Ding HL, Zhang LC (2014) Role of the cerebrospinal fluid-contacting nucleus in the descending inhibition of spinal pain transmission. Exp Neurol 261:475–485. https://doi.org/10.1016/j.expneurol.2014.07.018
Liu PF, Fang HZ, Yang Y, Zhang QQ, Zhou QQ, Chen SS, Zhou F, Zhang LC (2017) Activation of P2X3 receptors in the cerebrospinal fluid-contacting nucleus neurons reduces formalin-induced pain behavior via PAG in a rat model. Neurosci 358:93–102. https://doi.org/10.1016/j.neuroscience.2017.06.036
Montilla A, Mata GP, Matute C, Domercq M (2020) Contribution of P2X4 receptors to CNS function and pathophysiology. Int J Mol Sci 21:5562. https://doi.org/10.3390/ijms21155562
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. Academic, San Diego
Song S, Li Y, Bao C, Li Y, Yin P, Hong J, Li W, Shi Y, Zhang L (2019) Stereotaxic coordinates and morphological characterization of a unique nucleus (CSF-contacting nucleus) in rat. Front Neuroanat 13:47. https://doi.org/10.3389/fnana.2019.00047
Song S, Li Y, Zhai X, Li Y, Bao C, Shan C, Hong J, Cao J, Zhang L (2020) Monosynaptic input mapping of diencephalic projections to the cerebrospinal fluid-contacting nucleus in the rat. Front Neuroanat 14:7. https://doi.org/10.3389/fnana.2020.00007
Song S, Li Y, Zhai X, Li Y, Bao C, Shan C, Hong J, Cao J, Zhang L (2020) Connection input mapping and 3D reconstruction of the brainstem and spinal cord projections to the CSF-contacting nucleus. Front Neural Circuits 14:11. https://doi.org/10.3389/fncir.2020.00011
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nat 424:778–783. https://doi.org/10.1038/nature01786
Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K (2009) Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 5:28. https://doi.org/10.1186/1744-8069-5-28
Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci: Off J Soc Neurosci 28:11263–11268. https://doi.org/10.1523/JNEUROSCI.2308-08.2008
Vigh-Teichmann I, Vigh B (1983) The system of cerebrospinal fluid-contacting neurons. Arch Histol Jpn 46:427–468. https://doi.org/10.1679/aohc.46.427
Vigh B, Manzano e Silva MJ, Frank CL, Vincze C, Czirok SJ, Szabo A, Lukats A, Szel A (2004) The system of cerebrospinal fluid-contacting neurons. Its supposed role in the nonsynaptic signal transmission of the brain. Histol histopathol 19:607–628
Wang C, Wang H, Pang J, Li L, Zhang S, Song G, Li N, Cao J, Zhang L (2014) Glial cell-derived neurotrophic factor attenuates neuropathic pain in a mouse model of chronic constriction injury: possible involvement of E-cadherin/p120ctn signaling. J Mol Neurosci 54:156–163. https://doi.org/10.1007/s12031-014-0266-y
Wang CG, Song SY, Ding YL, Guo SQ, Liu X, Hao S, Li X, Chen N, Zhang Y, Zhang LC (2015) Extracellular signal-regulated kinase 5 in the cerebrospinal fluid-contacting nucleus contributes to neuropathic pain in rats. Pain Physician 18:E1073-1081
Wang QP, Nakai Y (1994) The dorsal raphe: an important nucleus in pain modulation. Brain Res Bull 34:575–585. https://doi.org/10.1016/0361-9230(94)90143-0
Wang XY, Yan WW, Zhang XL, Liu H, Zhang LC (2014) ASIC3 in the cerebrospinal fluid-contacting nucleus of brain parenchyma contributes to inflammatory pain in rats. Neurol Res 36:270–275. https://doi.org/10.1179/1743132813Y.0000000297
Lu X, Geng X, Zhang L, Zeng Y, Dong H, Yu H (2009) Substance P expression in the distal cerebrospinal fluid-contacting neurons and spinal trigeminal nucleus in formalin-induced the orofacial inflammatory pain in rats. Brain Res Bull 78:139–144. https://doi.org/10.1016/j.brainresbull.2008.11.011
Xing D, Wu Y, Li G, Song S, Liu Y, Liu H, Wang X, Fei Y, Zhang C, Li Y, Zhang L (2015) Role of cerebrospinal fluid-contacting nucleus in sodium sensing and sodium appetite. Physiol Behav 147:291–299. https://doi.org/10.1016/j.physbeh.2015.04.034
Yang R, Yang J, Li Z, Su R, Zou L, Li L, Xu X, Li G, Liu S, Liang S, Xu C (2022) Pinocembrin inhibits P2X4 receptor-mediated pyroptosis in hippocampus to alleviate the behaviours of chronic pain and depression comorbidity in rats. Mol Neurobiol 59:7119–7133. https://doi.org/10.1007/s12035-022-03023-x
Zabala A, Vazquez-Villoldo N, Rissiek B, Gejo J, Martin A, Palomino A, Perez-Samartín A, Pulagam K, Lukowiak M, Capetillo-Zarate E, Llop J, Magnus T, Koch-Nolte F, Rassendren F, Matute C, Domercq M (2018) P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med 10:e8743. https://doi.org/10.15252/emmm.201708743
Zhang C, Li Y, Wang X, Fei Y, Zhang L (2017) Involvement of neurokinin 1 receptor within the cerebrospinal fluidcontacting nucleus in visceral pain. Mol Med Rep 15:4300–4304. https://doi.org/10.3892/mmr.2017.6499
Zhang LC, Zeng YM, Ting J, Cao JP, Wang MS (2003) The distributions and signaling directions of the cerebrospinal fluid contacting neurons in the parenchyma of a rat brain. Brain Res 989:1–8. https://doi.org/10.1016/s0006-8993(03)03123-8
Zhang W, Hu D, Lin S, Fang X, Ye Z (2022) Contribution of P2X purinergic receptor in cerebral ischemia injury. Brain Res Bull 190:42–49. https://doi.org/10.1016/j.brainresbull.2022.09.009
Zhou F, Wang J, Zhang H, Liu H, Zhao G, Zu C, Lu X, Zhang L (2013) Evaluation of three tracers for labeling distal cerebrospinal fluid-contacting neurons. Neurosci Bull 29:576–580. https://doi.org/10.1007/s12264-013-1332-0
Zhou QQ, Chen SS, Zhang QQ, Liu PF, Fang HZ, Yang Y, Zhang LC (2017) Cerebrospinal fluid-contacting nucleus mediates nociception via release of fractalkine. Braz J Med Biol Res 50(9):e6275. https://doi.org/10.1590/1414-431x20176275
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81603700, 81703898 and 81904283) and Research project of Shanghai Municipal Health Commission (No.20194Y0307).
Author information
Authors and Affiliations
Contributions
JS, LZ, and WS: conception, supervision, and design of this article. WS, YY, and YZ: experiment and manuscript writing. LYL, GY, WT, and LLL: experiment and data collection. JG and JW: data analysis. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experiments were approved by the Committee for the Ethical Use of Laboratory Animals at Shanghai University of Traditional Chinese Medicine and conducted in accordance with the guidelines of the International Association for the Study of Pain (IASP).
Conflicts of interest
Wei Song declares that he/she has no conflict of interest.
Yue Yong declares that he/she has no conflict of interest.
Yalan Zhou declares that he/she has no conflict of interest.
Liyue Lu declares that he/she has no conflict of interest.
Guijie Yu declares that he/she has no conflict of interest.
Wei Tang declares that he/she has no conflict of interest.
Jian Wang declares that he/she has no conflict of interest.
Jun Guo declares that he/she has no conflict of interest.
Lili Li declares that he/she has no conflict of interest.
Licai Zhang declares that he/she has no conflict of interest.
Jiangang Song declares that he/she has no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, W., Yong, Y., Zhou, Y. et al. Activation of P2X4 receptors in midbrain cerebrospinal fluid-contacting nucleus leads to mechanical hyperalgesia in chronic constriction injury rats. Purinergic Signalling 19, 481–487 (2023). https://doi.org/10.1007/s11302-022-09911-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-022-09911-0