Abstract
Background
Osteosarcoma of the jaws is a rare primary malignant tumor of bone. The clinical, radiological and histopathological features of a case associated with cemento-osseous dysplasia is presented.
Case Report
A 57-year-old mixed-race female presented with a large, progressive, swelling of the right mandible. Radiographic examination revealed two associated lesions. Partially defined irregular radiopacities were noted in the left mandible, extending from the premolar to the molar region. The lesion had a cotton-wool appearance and resembled a fibro-osseous lesion; i.e. cemento-osseous dysplasia. A second large, expansive and irregular, radiopaque lesion was noted on the right angle of the mandible, extending beyond the inferior cortex of the mandible. The internal structure was heterogeneous and resembled irregular bone formation. The classic “sunburst” appearance of radiating bony spicules can be seen in the posterior–anterior view and the CBCT 3D reconstruction, indicating the outgrowth of the tumor matrix. Histopathological exam confirmed a final diagnosis of osteosarcoma closely associated with cemento-osseous dysplasia. The patient underwent a fludeoxyglucose-18 (FDG) positron emission tomography (PET) scan which indicated metastasis in the left lung and increased uptake in the right mandible. Chemotherapy was initially administered with a plan to resect the tumor, however, the patient demised as a result of medical complications.
Conclusion
The question in the literature remains whether these two entities are coincidentally found or arise from each other. Nevertheless, it is important for clinicians to closely monitor patients with cemento-osseous dysplasia and biopsy any suspicious lesions that may develop into osteosarcoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibro-osseous lesions (FOLs) refer to a group of conditions with shared histological features of irregular immature bone surrounded by a collagenous matrix. According to the latest 2022 World Health Organisation (WHO) classification, FOLs include the following: fibrous dysplasia, cemento-osseous dysplasia (COD), familial gigantiform cementoma, segmental odontomaxillary dysplasia, psammomatoid ossifying fibroma and juvenile trabecular ossifying fibroma [1].
COD is the most common benign fibro-osseous lesion (FOL) of the jaws and is generally considered to be self-limiting [1, 2]. As the name suggests, COD is of odontogenic origin and is limited to the tooth-bearing areas of the jaws. CODs embody a spectrum of related lesions that are said to arise from the periodontal ligament [3]. While all variants of COD represent the same histopathological process, they are distinguished based on their clinical and radiographic features. There are three subtypes based on anatomical location and jawbone involvement: periapical, focal and florid [2, 3].
COD has a predilection for middle-aged women of African descent [2, 4, 5]. Radiologically, lesions undergo three stages: lucent, mixed and opaque depending on the progression of mineralization. A “cotton wool” appearance is frequently reported [2, 4, 5]. Typically, COD is asymptomatic, can be diagnosed radiologically and does not require histological confirmation or specific treatment. Some have reported biopsy to be contraindicated as it results in complications such as persistent local infection. In most instances, surgical removal is warranted in secondarily infected lesions and those exposed to the oral environment [2, 3, 6].
In contrast, osteosarcoma (OS) of the jaws is a rare malignant tumor of bone, accounting for only 6% of all OS [1, 7]. OS is characterised by neoplastic cells producing osteoid or bone. One of the most characteristic symptoms of OS is paraesthesia, pain and swelling of the jaw [8, 9]. Radiologically, OS is described as an ill-defined, osteolytic, and ragged radiolucency. It can also result in new periosteal bone formation, perpendicular to the existing cortical plate known as the “sunburst/sunray appearance” [4, 6, 9]. It is interesting to note that OS can arise within FOLs such as Paget’s disease and fibrous dysplasia and in persons exposed to radio therapy [10].
The association of OS with COD is rare. This case report represents a possible association between benign and malignant entities. The literature regarding clinicopathologic features, differential diagnosis and management is also reviewed. Before detailing the clinical presentation and treatment, we confirm that signed informed consent was obtained from the individual participant for whom identifying information is included in this article.
Case report
A 57-year-old mixed-race female was referred to the Oral and Maxillofacial Surgery outpatient clinic. She presented with the main complaint of a large outgrowth in her mouth with a history of approximately 2 months (Fig. 1). The patient had root remnants removed in this region 2 months before the swelling started. According to the patient, it was increasing in size, painful and interfering with daily oral activities. Apart from a positive history of hypertension and rheumatoid arthritis, the medical history revealed no other medical conditions or allergies. Upon clinical examination, a large palpable facial swelling on the right side of the mandible was noted and no lymphadenopathy was detected.
Radiology
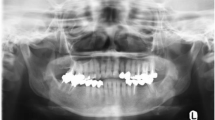
A digital panoramic radiograph (DPR) and a posterior-anterior (PA) skull radiograph (Figs. 2, 3) showed two coinciding lesions in the mandible. Partially defined irregular radiopacities were noted in the 3rd quadrant; extending from the premolar to the molar region. The lesion had a cotton-wool appearance. Radiologically, the lesion resembled a fibro-osseous nature; i.e. COD.
DPR shows partially defined irregular radiopacities in the left mandible within the premolar-molar region. The lesion displays a cotton-wool appearance (open arrow). A large expansive and irregular radiopaque lesion was noted on the right angle of the mandible, extending beyond the inferior cortex of the mandible (solid white arrow)
A second large, expansive and irregular, radiopaque lesion was noted on the right angle of the mandible, extending beyond the inferior cortex of the mandible. The borders were poorly defined. The internal structure was heterogeneous/mixed and appeared to resemble bone formation. The inferior alveolar canal was partially traceable on this view. A CBCT and computed tomography (CT) scans were performed, and the volumes were evaluated in all three planes (Fig. 4). Contrast CT and positron emission tomography (PET) scans were also performed showing the extent of the lesion and increased uptake in the right mandible, respectively (Fig. 5). A sunburst appearance of radiating bony spicules and accompanying soft tissue swelling can be seen indicating outgrowth of the tumor matrix.
a CBCT axial slice, b CT axial slice; shows bone formation within the lesion at the right mandible (solid arrow). A cotton wool appearance is seen in the right mandible (dashed arrow), closely related to the OS. A mixed density is seen in the left mandible (open arrow). c CBCT sagittal oblique slice shows a root remnant in the left mandible with a periapical low-density (dashed arrow); inferiorly there is a mixed heterogenous lesion. d CBCT coronal slice shows an intermediate heterogenous lesion in the left mandible causing lateral expansion (solid arrow). e CBCT coronal slice, f CT coronal slice; bone formation radiation from the lingual aspect of the right mandible; buccal soft tissue swelling also evident (solid arrows)
a CT axial slice, b CT axial slice with contrast, c CT coronal slice, d CT coronal slice with contras. a–d: The sclerotic bone lesion has a sunburst appearance arising from the inner cortex of the mandible with associated heterogeneously enhancing mass which extends to the masticator, buccal and carotid spaces. There is erosion of the posterior wall of the right maxillary sinus. Mass effect on the tongue displacing the tongue to the left. e PET scan—axial, f PET scan—coronal. e–f: Increased uptake noted in the anterior and medial aspect of the lesion
Specifically for this case, the differential diagnosis included the association of a malignant and benign lesion; namely OS, with a FOL, i.e. COD. The diagnosis of COD was made radiologically only.
Histopathology
An incisional biopsy revealed a malignant neoplasm with epithelioid to oval cells arranged in sheets and lobules. Nuclear features varied from hyperchromatic to vesicular with prominent nucleoli. The cytoplasm was eosinophilic to clear, with irregular cell borders, in a background of variable amounts of immature osteoid deposition and frequent mitoses (Fig. 6). Necrosis in this biopsy specimen was absent. Immunohistochemistry (SATB2) supported the diagnosis of an OS, after other malignant tumors, including carcinomas and lymphomas, were excluded.
a High magnification demonstrating malignant epithelioid cells arranged in lobules, with hyperchromatic nuclei and prominent nucleoli. Prominent mitotic figures are also demonstrated (× 400) b Lace-like malignant osteoid at high magnification (× 400), c Periodic-Acid Schiff highlighting the lace-like osteoid (× 200)
Management
The patient underwent fludeoxyglucose-18 (FDG) PET scan which showed perineural invasion of the right inferior alveolar nerve as well as pulmonary nodules in the left upper lobe and left supraclavicular lymph node. Upon review of the patient at the multidisciplinary meeting, the treatment plan involved the administration of neoadjuvant chemotherapy with surgical resection of the tumor. The patient received three doses of chemotherapy and then demised from medical complications prior to receiving any surgical intervention.
Discussion
FOLs of the craniomaxillofacial region are described as the replacement of bone by cellular fibrous tissue containing varied amount of mineralized tissue. The lesions share a similar histological appearance, thus clinical and radiological features must be correlated to determine a final diagnosis [7, 11]. FOLs usually present a favourable prognosis, but evidence suggests that some FOLs, especially fibrous dysplasia, may have a risk of spontaneous malignant transformation [11].
COD is the most common FOL and has a predilection for middle-aged women of African descent [3, 4, 9]. As the name suggests, it is limited to the tooth-bearing region; characterized by the replacement of bone with cementum-like material [3, 6, 7]. These lesions lack expansile growth, are asymptomatic and normally detected as incidental findings on routine radiographic examination [2].
COD is further divided into three categories: periapical cemental dysplasia (PCD), focal COD and florid COD. PCD is a localized variant affecting the apices of vital mandibular anterior teeth. The focal variant is a solitary lesion usually not associated with a tooth and florid COD involves two or more quadrants of the jaws [5, 9].
Radiologically, as COD matures it transforms between three stages depending on the degree of mineralization, namely: (1) lucent/early stage where normal bone is resorbed, (2) mixed stage followed by (3) the opaque/final phase. New bone is deposited in increasing amounts in the second and third stages [5, 9, 12].
The interpretation of COD can be made based on the radiologic features and clinical findings [13]. Since COD can be diagnosed radiologically, it does not always require histological confirmation [2]. Due to the decreased vascularity in COD, biopsies are controversial as they predisposes the patient to osteomyelitis [2, 3, 14, 15]. CODs usually do not require treatment, but due to the tendency for mature lesions to become secondarily infected or exposed in the oral cavity, surgical intervention is then required to avoid complications such as osteomyelitis or draining cutaneous fistula [13].
Turning to OS, which in contrast to COD, is a malignant tumor of bone with a sinister prognosis. Symptoms such as pain and swelling are characteristic [6]. The case reported by Schneider et al. was the first example of a sarcoma arising in association with florid osseous dysplasia [15]. The concurrence of COD and OS is rare and, therefore, sparsely reported in the literature. There are only six reported cases known to the authors. A review of these cases was conducted, and a comparison is summarized in Table 1 [2, 6, 15,16,17]. The concurrence of COD and OS, as noted in these cases, prompts a re-evaluation of potential pathophysiological links. Wagner et al. has suggested that certain fibro-osseous lesions may harbour malignant potential under specific conditions [11]. Furthermore, genetic studies have identified pathways that could be implicated in both COD and OS, suggesting a relationship that may extend beyond mere coincidence [10]. The cases outlined in Table 1 demonstrate a range of demographic profiles and clinical presentations, yet consistently show an association between COD and the subsequent development of OS in the mandible. For instance, Schneider et al. and Melrose and Handlers both reported cases from the USA where patients presented with clinical and radiographic features suggestive of both COD and OS in the mandible [15, 16]. Lopes et al., Olusanya et al. and Haefliger et al., noted similar findings in Brazil, Nigeria and Switzerland respectively, indicating that this association is observed across different populations and geographic locations [2, 6, 17]. Haefliger et al. presented a case of a patient with established COD who developed high-grade OS over a period of 9 years [2]. This longitudinal evidence supports the hypothesis that COD may, under certain conditions, undergo malignant transformation. The review of these literatures supports a hypothesis of related pathogenesis, warranting further molecular and epidemiological studies.
OS of the jaws is rare and clinically distinct from OS of the long bones [2, 18], with the latter being diagnosed amongst adolescents and the former presenting between the 4th–5th decades [9]. Males and females are said to be equally affected. Paraesthesia, pain, loosening of teeth, swelling and ulceration are the most common symptoms present. Previous radiation therapy, fibrous dysplasia, Paget's disease are known pre-disposing factors [9, 12].
OS presents radiographically as an osteolytic lesion. Mineralization of the tumor matrix is frequently seen and depending on the stage of mineralization, it can appear radiolucent, mixed, or radiopaque. Several periosteal reactions have been described in the literature. These include Codman’s triangle; seen at the periphery of elevated periosteum, and the characteristic “sunburst” appearance [4, 9, 12, 19]. These features are seen in mandibular tumors [9].
Pathogenesis and histopathology
Many OS arise spontaneously, however, there may be an increased risk of developing secondary OS with prior radiotherapy, an underlying Paget’s disease of bone, pre-existing bone lesions, or genetic predispositions (e.g. Li-Fraumeni syndrome). Molecular alterations include TP53 gene and retinoblastoma gene pathway alterations, translocations and numerical alterations. Cell cycle regulators, including CDK4 and CDKN2A genes, may also be altered in some cases [20]. The jaw bones are more commonly affected in the head and neck than any other craniofacial bones The majority of OS in the gnathic bones occurs intramedullary, but cases of parosteal types have been reported [21].
Conventional OS is characterised by atypical cells producing neoplastic, lace-like osteoid. The cells may be polygonal or epithelioid in the osteoblastic variant, similar to the case described in this report. The matrix may demonstrate extensive variability, including heavy mineralised sclerotic bone. Higher-grade OS are more aggressive, resulting in extensive bone destruction. There are several histologic subtypes, classified according to the predominant matrix, including osteoblastic, chondroblastic and fibroblastic. Immunohistochemical staining to confirm osteoblastic differentiation includes SATB2, MDM2 and CDK4 [21].
To date, and to the authors’ best knowledge, six cases have been reported where patients with COD also developed OS [2, 6]. One of these patients was known with Li-Fraumeni Syndrome, which predisposes those affected to developing malignant tumors due to TP53 mutations [2]. The molecular pathogenesis of COD is not well known but is not considered a precursor lesion for OS [2].
Diagnosis and management
Management of any FOLs suspected to have developed malignancy is an urgent incisional biopsy to confirm the diagnosis. Histopathological confirmed malignancies should be discussed in a multi-disciplinary tumor board to establish the best care for the patient. OS are predominantly managed with an initial period of chemotherapy followed by wide surgical resection unless the patient is deemed more suitable for palliation [18].
Wagner et al., performed a systematic review of the literature concerning all documented cases of malignant transformation of craniomaxillofacial fibro‐osseous lesions [11]. Their results revealed that fibrous dysplasia was the only FOL that seemed to have a significant risk of malignant transformation; with OS being the most common malignant tumor [11]. It is advised that the initial diagnosis of any FOL be substantiated by clinical, radiological, and histopathological evaluation since some cases can progress to OS or lead to a misdiagnosis [11].
The association of OS and COD raises the question of whether a collision tumor has resulted in the transformation of COD into OS or their simultaneous presence is a mere coincidence [6, 15]. There is no consensus in the literature about the potential for malignant transformation of COD. Based on the literature reviewed, and this individual case, we cannot conclusively establish causation, but the co-occurrence of these conditions suggests more than a coincidental relationship. It highlights a potential pathogenic link, warranting further investigation. Whilst this paucity exists in the literature and the risk of malignant transformation may be even less, it should not be considered negligible. Awareness of possible malignant transformation can guide healthcare professionals to establish more stringent follow-ups. This is especially true for lesions such as COD as it is rarely surgically treated.
References
Vered M, Wright JM. Update from the 5th edition of the world health organization classification of head and neck tumors odontogenic and maxillofacial bone tumours. Head Neck Pathol [Internet]; 2022. https://doi.org/10.1007/s12105-021-01404-7.
Haefliger S, Harder D, Kovac M, Linkeschova K, Eufinger H, Baumhoer D. Osteosarcoma of the mandible in a patient with florid cemento-osseos dysplasia and li–fraumeni syndrome: a rare coincidence. Head Neck Pathol [Internet]. 2021;15(2):704–8. https://doi.org/10.1007/s12105-020-01223-2.
Fenerty S, Shaw W, Verma R, Syed AB, Kuklani R, Yang J, et al. Florid cemento-osseous dysplasia: review of an uncommon fibro-osseous lesion of the jaw with important clinical implications. Skeletal Radiol. 2017;46(5):581–90.
Langlais RP, Langland OE, Nortje CJ. Diagnostic imaging of the jaws. 1st ed. Malvern: Williams & Wilkins; 1995.
Farman AG, Nortje CJ, Wood RE. Fibro-osseous Lesions. In: Oral and maxillofacial diagnostic imaging. 1st ed. St Louis: Mosby; 1993. p. 319.
Olusanya AA, Adeyemi BF, Adisa AO. Concurrent cemento-osseous dysplasia and osteogenic sarcoma: report of two cases. Case Rep Med. 2012. https://doi.org/10.1155/2012/180561.
Baumhoer D, Haefliger S, Ameline B, Hartmann W, Amary F, Cleven A, et al. Ossifying fibroma of non-odontogenic origin: a fibro-osseous lesion in the craniofacial skeleton to be (re-)considered. Head Neck Pathol [Internet]. 2022;16(1):257–67. https://doi.org/10.1007/s12105-021-01351-3.
Li B-B, Zhang J-Y, Gao Y. Chondroblastic osteosarcoma arising in the maxilla mimicking the radiographic and histological characteristics of cemento-osseous lesions: a case report. Mol Clin Oncol. 2017;6(5):733–6.
Hameed M, Horvai AE, Jordan RCK. Soft tissue special issue: gnathic fibro-osseous lesions and osteosarcoma. Head Neck Pathol [Internet]. 2020;14:70–82.
Savage SA, Mirabello L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma. 2011;2011:14–6.
Wagner VP, Carlos R, Romañach MJ, Lopes MA, Speight PM, Vargas PA. Malignant transformation of craniomaxillofacial fibro-osseous lesions: a systematic review. J Oral Pathol Med. 2019;48(6):441–50.
Lam EWN. Malignant neoplasms. In: Mallya SM, Lam EWN, editors. White & pharoah’s oral radiology: principles and interpretation. 8th ed. St Louis: Elsevier; 2019. p. 493–504.
Lam EWN. Diseases affecting the structure of bone. In: Mallya SM, Lam EWN, editors. White & pharoah’s oral radiology: principles and interpretation. 8th ed. St Louis: Elsevier; 2019. p. 475–85.
Singer SR, Mupparapu M, Rinaggio J. Florid cementoosseous dysplasia and chronic diffuse osteomyelitis report of a simultaneous presentation and review of the literature. J Am Dent Assoc Internet. 2005;136(7):927–31.
Schneider LC, Dolinsky HB, Grodjesk JE, Mesa ML, Doyle JL. Malignant spindle cell tumor arising in the mandible of a patient with florid osseous dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(1):69–73.
Melrose R, Handlers J. Osteosarcoma ex florid osseous dysplasia: report of a case. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology. 2003;96(September):296.
Lopes MA, Kim HS, Mariano FV, Corrêa MB, de Rabelo NTA, Vargas PA. Clinico-pathologic conference: case 1. Head Neck Pathol. 2010;4(4):329–33.
Lee RJ, Arshi A, Schwartz HC, Christensen RE. Characteristics and prognostic factors of osteosarcoma of the jaws a retrospective cohort study. JAMA Otolaryngol—Head Neck Surg. 2015;141(5):470–7.
Padilla RJ, Murrah VA. The spectrum of gnathic osteosarcoma: caveats for the clinician and the pathologist. Head Neck Pathol. 2011;5(1):92–9.
Reith JD. Bone and Joints. In: Goldblum JR, Lamps LW, McKenney JK, Myers JL, editors. Rosai and ackerman’s surgical pathology. 11th ed. New York: Elsevier; 2003. p. 1752–9.
Lopes M, Baumhoer D, Jay A. Osteosarcoma of the jaw. In: WHO classification of head and neck tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2022. p. 224.
Acknowledgements
Marlise Coetzee—Administrator of the Diagnostic Radiology Museum at the Faculty of Dentistry, University of the Western Cape. Bartho Siebrits—Clinical Photographer, Faculty of Dentistry, University of the Western Cape.
Funding
Open access funding provided by University of the Western Cape. This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent
Additional informed consent was obtained from the patient for which identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Indermun, S., Titinchi, F., Alwan, J. et al. Osteosarcoma associated with cemento-osseous dysplasia: co-incidence or two related entities?. Oral Radiol (2024). https://doi.org/10.1007/s11282-024-00758-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11282-024-00758-2