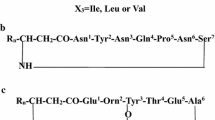Abstract
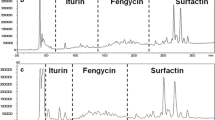
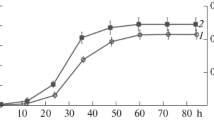
Bacterial lipopeptides have become a research focus of many studies owing to their industrial and pharmaceutical importance. Although such studies focused on researching purification procedures and qualitative analysis, much remains to be explored and developed to improve the current methods. To enable thorough studies of lipopeptides, this paper describes a new method for purification and characterization of in-gel anionic lipopeptides. Specifically, lipopeptides attributed to the anti-staphylococcal activity of Bacillus mojavensis HF were separated using SDS-PAGE (sodium dodecyl sulphate–polyacrylamide gel electrophoresis) and subsequently characterized using mass spectrometry. Lipopeptide band obtained by gel electrophoresis was first visualized using three different staining methods. Next, the lipopeptide isomers were efficiently recovered from the gel band and structural characterization of the extracted lipopeptides was carried out by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). MS analysis revealed that Bacillus mojavensis HF produced three types of lipopeptides including surfactin, fengycin, and kurstakin. 14 clusters of ion peaks were identified as fengycin A with fatty acid of C15-C17, fengycin B (C16, C17), surfactin (C13-C16), and kurstakin (C9-C12). Moreover, tandem mass spectrometric analysis (MS/MS) revealed the sequences of fengycin A and surfactin. In this study, we identified a high variety and number of surfactin and fengycin isomers, which previous reports lacked. To the best of our knowledge, we are the first to report the presence of kurstakin in Bacillus mojavensis species. Finally, we demonstrated that our gel-based study of lipopeptides allowed for a precise and reproducible investigation of these molecules.







Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
References
Alajlani M, Shiekh A, Hasnain S, Brantner A (2016) Purification of bioactive lipopeptides produced by Bacillus subtilis strain BIA. Chromatographia 79:1527–1532
Andrews AT (1986) Electrophoresis: theory, techniques and biochemical and clinical applications. Clarendon Press, New York
Béchet M, Caradec T, Hussein W, Abderrahmani A, Chollet M, Leclère V, Dubois T, Lereclus D, Pupin M, Jacques P (2012) Structure, biosynthesis, and properties of kurstakins, nonribosomal lipopeptides from Bacillus spp. Appl Microbiol Biot 95:593–600
Bie X, Lu Z, Lu F (2009) Identification of fengycin homologues from Bacillus subtilis with ESI-MS/CID. J Microbiol Methods 79:272–278
Breil C, Abert Vian M, Zemb T, Kunz W, Chemat F (2017) “Bligh and Dyer” and Folch methods for solid–liquid–liquid extraction of lipids from microorganisms. Comprehension of solvatation mechanisms and towards substitution with alternative solvents. Int J Mol Sci. https://doi.org/10.3390/ijms18040708
Chen HL, Lee YS, Wei YH, Juang RS (2008) Purification of surfactin in pretreated fermentation broths by adsorptive removal of impurities. Biochem Eng J. https://doi.org/10.1016/j.bej.2008.01.020
Chen WC, Juang RS, Wei YH (2015) Applications of a lipopeptide biosurfactant, surfactin, produced by microorganisms. Biochem Eng J. https://doi.org/10.1016/j.bej.2015.07.009
de Faria AF, Stéfani D, Vaz BG, Silva ÍS, Garcia JS, Eberlin MN, Grossman MJ, Alves OL, Durrant LR (2011) Purification and structural characterization of fengycin homologues produced by Bacillus subtilis LSFM-05 grown on raw glycerol. J Ind Microbiol Biotechnol 38:863–871
de Moreno MR, Smith JF, Smith RV (1986) Mechanism studies of coomassie blue and silver staining of proteins. J Pharm Sci 75:907–911
Dehghanifar S, Keyhanfar M, Emtiazi G (2019) Production and partial purification of thermostable bacteriocins from Bacillus pumilus ZED17 and DFAR8 strains with antifungal activity Mol. Biol Res Commun 8:41
Dimkić I, Stanković S, Nišavić M, Petković M, Ristivojević P, Fira D, Berić T (2017) The profile and antimicrobial activity of Bacillus lipopeptide extracts of five potential biocontrol strains. Front Microbiol 8:925
Dlamini B (2017) Downstream purification of surfactin produced by Bacillus subtilis ATCC 21332. Stellenbosch University, Stellenbosch
Fanaei M, Emtiazi G (2018) Microbial assisted (Bacillus mojavensis) production of bio-surfactant lipopeptide with potential pharmaceutical applications and its characterization by MALDI-TOF-MS analysis. J Mol Liq. https://doi.org/10.1016/j.molliq.2018.07.103
Grangemard I, Peypoux F, Wallach J, Das BC, Labbé H, Caille A, Genest M, Maget-Dana R, Ptak M, Bonmatin JM (1997) Lipopeptides with improved properties: structure by NMR, purification by HPLC and structure–activity relationships of new isoleucyl-rich surfactins. J Pept Sci 3:145–154
Hathout Y, Ho YP, Ryzhov V, Demirev P, Fenselau C (2000) Kurstakins: a new class of Lipopeptides isolated from Bacillus thuringiensis. J Nat Prod 63:1492–1496
Kambourova M, Tangney M, Priest FG (2001) Regulation of polyglutamic acid synthesis by glutamate in Bacillus licheniformis and Bacillus subtilis. Appl Environ Microbiol 67:1004–1007
Kim PI, Ryu J, Kim YH, Chi YT (2010) Production of biosurfactant lipopeptides Iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol 20:138–145
Kowall M, Vater J, Kluge B, Stein T, Franke P, Ziessow D (1998) Separation and characterization of surfactin isoforms produced by Bacillus subtilis OKB 105. J Colloid Interface Sci 204:1–8
Li XY, Mao ZC, Wang YH, Wu YX, He YQ, Long CL (2012) ESI LC-MS and MS/MS characterization of antifungal cyclic lipopeptides produced by Bacillus subtilis XF-1. J Mol Microbiol Biotechnol 22:83–93
Li X, Zhang Y, Wei Z, Guan Z, Cai Y, Liao X (2016) Antifungal activity of isolated Bacillus amyloliquefaciens SYBC H47 for the biocontrol of peach gummosis. PloS One 11:e0162125
Liu Y, Teng K, Wang T, Dong E, Zhang M, Tao Y, Zhong J (2020) Antimicrobial Bacillus velezensis HC6: production of three kinds of lipopeptides and biocontrol potential in maize. J Appl Microbiol 128:242–254
Ma Z, Wang N, Hu J, Wang S (2012) Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J Antibiot 65:317–322
Meena KR, Kanwar SS (2015) Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. Biomed Res Int. https://doi.org/10.1155/2015/473050
Moyne AL, Shelby R, Cleveland T, Tuzun S (2001) Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. J Appl Microbiol 90:622–629
Naughton P, Marchant R, Naughton V, Banat I (2019) Microbial biosurfactants: current trends and applications in agricultural and biomedical industries. J Appl Microbiol 127:12–28
Pathak KV, Keharia H (2013) Characterization of fungal antagonistic bacilli isolated from aerial roots of banyan (F icus benghalensis) using intact-cell MALDI-TOF mass spectrometry (ICMS). J Appl Microbiol 114:1300–1310
Pathak KV, Bose A, Keharia H (2014) Characterization of novel lipopeptides produced by Bacillus tequilensis P15 using liquid chromatography coupled electron spray ionization tandem mass spectrometry (LC–ESI–MS/MS). J Pept Res Ther 20:133–143
Prat J, Lamy J, Weill J (1969) Staining of lipoproteins after electrophoresis in polyacrylamide gel. Bulletin de la Societe de Chimie Biologique 51:1367–1367
Roy A, Mahata D, Paul D, Korpole S, Franco OL, Mandal SM (2013) Purification, biochemical characterization and self-assembled structure of a fengycin-like antifungal peptide from Bacillus thuringiensis strain SM1. Front Microbiol 4:332. https://doi.org/10.3389/fmicb.2013.00332
Sa RB, An X, Sui JK, Wang XH, Ji C, Wang CQ, Li Q, Hu YR, Liu X (2018) Purification and structural characterization of fengycin homologues produced by Bacillus subtilis from poplar wood bark. Australas Plant Path 47:259–268
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold spring harbor laboratory press
Sharma D, Mandal SM, Manhas RK (2014) Purification and characterization of a novel lipopeptide from Streptomyces amritsarensis sp. Nov. active against methicillin-resistant Staphylococcus aureus. AMB Express 4:1–9
Smyth T, Perfumo A, McClean S, Marchant R, Banat I (2010) Isolation and Analysis of Lipopeptides and High Molecular Weight Biosurfactants. In: Handbook of hydrocarbon and lipid microbiology. pp 3688–3704
Snook ME, Mitchell T, Hinton DM, Bacon CW (2009) Isolation and characterization of Leu7-surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. J Agric Food Chem 57:4287–4292
Uttlová P, Pinkas D, Bechyňková O, Fišer R, Svobodová J, Seydlová G (2016) Bacillus subtilis alters the proportion of major membrane phospholipids in response to surfactin exposure. Biochimica et Biophysica Acta -Biomembranes 1858:2965–2971
Vaisar T (2009) Thematic review series: proteomics. Proteomic analysis of lipid-protein complexes. J Lipid Res 50:781–786
Waewthongrak W, Leelasuphakul W, McCollum G (2014) Cyclic lipopeptides from Bacillus subtilis ABS–S14 elicit defense-related gene expression in citrus fruit. PloS One. https://doi.org/10.1371/journal.pone.0109386
Zhi Y, Wu Q, Xu Y (2017) Genome and transcriptome analysis of surfactin biosynthesis in Bacillus amyloliquefaciens MT45. Sci Rep. https://doi.org/10.1038/srep40976
Acknowledgements
The authors wish to thank the University of Isfahan for financial support of this work.
Funding
The current study was supported by grant from the University of Isfahan to Maryam Fanaei for obtaining PhD degree.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fanaei, M., Jurcic, K. & Emtiazi, G. Detection of simultaneous production of kurstakin, fengycin and surfactin lipopeptides in Bacillus mojavensis using a novel gel-based method and MALDI-TOF spectrometry. World J Microbiol Biotechnol 37, 97 (2021). https://doi.org/10.1007/s11274-021-03064-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03064-9