Abstract
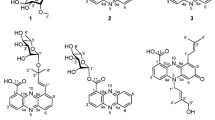
Polyketides and peptides obtained from actinobacteria are important therapeutic compounds which include front line antibiotics and anticancer drugs. Many screening programs are directed towards isolation of bioactive compounds from these organisms but the chances of finding novel antimicrobial leads among common actinobacteria are fast dwindling. As a result, the focus has shifted to the members of less exploited genera of rare actinobacteria. Three isolates, MMS8, MMS16 and KCR3 found to be potent polyketide and peptide producers were identified by 16S rRNA gene sequencing and their sequences deposited in the GenBank under the accession numbers MG407702, MG372012 and MG430204 respectively. MMS8 identified as Micromonospora auratinigra, yielded one potent compound determined to be chloroanthraquinone with an minimum inhibitory concentration (MIC) of 8 µg/ml against Bacillus subtilis and an IC50 value of 10 µg/ml and 4 μg/ml against HeLa and IMR cell lines respectively. This is the first report of the production of chloroanthraquinone by M. auratinigra. MMS16, identified as a member of the family Micromonosporaceae, yielded a potent compound MMS16B analyzed to be a novel bafilomycin analogue. The MIC of the compound was found to be 7 μg/ml against B.subtilis and IC50 value against HeLa and IMR was observed to be 9 μg/ml and 14 μg/ml respectively. MMS16B was also found to exhibit anti-quorum sensing (AQS) activity at sublethal concentrations. KCR3 identified as Kocuria kristinae yielded a novel antimicrobial peptide with antibacterial, antifungal and AQS activity. To the best of our knowledge, no antimicrobial activity has ever been reported from K. kristinae.







Similar content being viewed by others
References
Anderson AS, Wellington EM (2001) The taxonomy of Streptomyces and related genera. Int J Syst Evol Microbiol 51(3):797–814
Arthington-Skaggs BA, Lee-Yang W, Ciblak MA, Frade JP, Brandt ME, Hajjeh RA, Harrison LH, Sofair AN, Warnock DW, Candidemia Active Surveillance Group (2002) Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob Agents Chemother 46(8):2477–2481
Augustine SK, Bhavsar SP, Kapadnis BP (2005) A non-polyene antifungal antibiotic from Streptomyces albidoflavus PU 23. J Biosci 30(2):201–211
Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Klenk HP, Clément C, Ouhdouch Y, van Wezel GP (2016) Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev 80(1):1–43
Begde D, Bundale S, Mashitha P, Rudra J, Nashikkar N, Upadhyay A (2011) Immunomodulatory efficacy of nisin—a bacterial lantibiotic peptide. J Pept Sci 17(6):438–444
Bundale SB, Begde DN, Nashikkar NA, Kadam TA, Upadhyay AU (2015) Optimization of culture conditions for production of bioactive metabolites by Streptomyces spp. isolated from soil. Adv Microbiol 5(06):441–451
Bundale SB, Begde DN, Pillai D, Gangwani K, Nashikkar NA, Kadam TA, Upadhyay AU (2018a) Novel aromatic polyketides from soil Streptomyces spp.: purification, characterization and bioactivity studies. World J Microbiol Biotechnol 34:1–6
Bundale SB, Singh J, Begde DN, Nashikkar NA, Upadhyay AU (2018b) Culturable rare actinomycetes from Indian forest soils: molecular and physicochemical screening for biosynthetic genes. Iran J Microbiol 10(2):132–142
Dharmaraj S (2011) Antagonistic potential of marine Actinobacteria against fish and shellfish pathogens. Turk J Biol 35(3):303–311
Ding Y, Huang Y, Ruan J, Gao Y (2009) Selective isolation and diversity of acidophilic filamentous actinomycetes from acidic soils. Wei sheng wu xue bao = Acta microbiol Sin 49(6):710–717
Ding D, Chen G, Wang B, Wang Q, Liu D, Peng M, Shi P (2013) Culturable actinomycetes from desert ecosystem in northeast of Qinghai-Tibet Plateau. Ann Microbiol 63(1):259–266
Fabian H, Mäntele W (2006) Infrared spectroscopy of proteins. In: Handbook of vibrational spectroscopy. Wiley Online Library. https://doi.org/10.1002/0470027320.s8201
Hamedi J, Imanparast S, Mohammadipanah F (2015) Molecular, chemical and biological screening of soil actinomycete isolates in seeking bioactive peptide metabolites. Iran J Microbiol 7(1):23
Igarashi Y, Trujillo ME, Martínez-Molina E, Yanase S, Miyanaga S, Obata T, Sakurai H, Saiki I, Fujita T, Furumai T (2007) Antitumor anthraquinones from an endophytic actinomycete Micromonospora lupini sp. nov. Bioorg Med Chem Lett 17(13):3702–3705
Johdo O, Watanabe Y, Ishikura T, Yoshimoto A, Naganawa H, Sawa T, Takeuchi T (1991) Anthracycline metabolites from Streptomyces violaceus A262. J Antibiot 44(10):1121–1129
Kanagasabhapathy M, Yamazaki G, Ishida A, Sasaki H, Nagata S (2009) Presence of quorum-sensing inhibitor-like compounds from bacteria isolated from the brown alga Colpomenia sinuosa. Lett Appl Microbiol 49:573–579
Kim HS, Hong YS, Kim YH, Yoo OJ, Lee JJ (1996) New anthracycline metabolites produced by the aklavinone 11-hydroxylase gene in Streptomyces galilaeus ATCC 31133. J Antibiot 49(4):355–360
Kurtböke DI (2012) Biodiscovery from rare actinomycetes: an eco-taxonomical perspective. Appl Microbiol Biotechnol 93(5):1843–1852
Laakso JA, Mocek UM, Van Dun J, Wouters W, Janicot M (2003) R176502, a new bafilolide metabolite with potent antiproliferative activity from a novel Micromonospora species. J Antibiot 56(11):909–916
Martín J, da Sousa ST, Crespo G, Palomo S, González I, Tormo JR, de la Cruz M, Anderson M, Hill RT, Vicente F, Genilloud O (2013) Kocurin, the true structure of PM181104, an anti-methicillin-resistant Staphylococcus aureus (MRSA) thiazolyl peptide from the marine-derived bacterium Kocuria palustris. Mar drugs 11(2):387–398
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Nashikkar NA, Begde DN, Bundale SB, Pise MV, Rudra JA, Upadhyay AU (2011) Inhibition of swarming motility, biofilm formation and virulence factor expression of urinary pathogens by Euphorbia trigona latex extracts. Int J Pharm Sci Res 2(3):558
Omura S, Otoguro K, Nishikiori T, Oiwa R, Iwai Y (1981) Setamycin, a new antibiotic. J Antibiot 34(10):1253–1256
Osman CP, Weber JF, Ismail NH (2014) UV/visible spectra of a series of natural and synthesised anthraquinones: experimental and quantum chemical approaches. SpringerPlus 3(1):233. https://doi.org/10.1186/2193-1801-3-233
Otoguro K, Nakagawa A, Omura S (1988) Setamycin, a 16-membered macrolide antibiotic identification and nematocidal activity. J Antibiot 41(2):250–252
Pridmore D, Rekhif N, Pittet AC, Suri B, Mollet B (1996) Variacin, a new lanthionine-containing bacteriocin produced by Micrococcus varians: comparison to lacticin 481 of Lactococcus lactis. Appl Environ Microbiol 62(5):1799–1802
Rajanbabu V, Chen JY (2011) Applications of antimicrobial peptides from fish and perspectives for the future. Peptides 32(2):415–420
Rosso ML, Bertoni MD, Adler MT, Maier MS (2003) Anthraquinones from the cultured lichen mycobionts of Teloschistes exilis and Caloplaca erythrantha. Biochem Syst Ecol 31(10):1197–1200
Shen B (2003) Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr Opin Chem Biol 7(2):285–295
Silverstein RM, Webster FX, Kiemle DJ, Bryce DL (2014) Spectrometric identification of organic compounds. Wiley, Hoboken
Talukdar M, Bordoloi M, Dutta PP, Saikia S, Kolita B, Talukdar S, Nath S, Yadav A, Saikia R, Jha DK, Bora TC (2016) Structure elucidation and biological activity of antibacterial compound from Micromonospora auratinigra, a soil Actinomycetes. J Appl Microbiol 121(4):973–987
Tiwari K, Gupta RK (2012) Rare actinomycetes: a potential storehouse for novel antibiotics. Crit Rev Biotechnol 32(2):108–132
Werner G, Hagenmaier H, Drautz H, Baumgartner A, Zahner H (1984) Metabolic products of microorganisms. 224. J Antibiot 37(2):110–117
Xue CM, Tian L, Lin WH, Deng ZW (2009) Anthraquinone derivatives from Micromonospora rhodorangea. Nat Prod Res 23(6):533–538
Zhao P, Xue Y, Gao W, Li J, Zu X, Fu D, Feng S, Bai X, Zuo Y, Li P (2018) Actinobacteria-derived peptide antibiotics since 2000. Peptides. https://doi.org/10.1016/j.peptides.2018.03.011
Acknowledgements
The authors would like to acknowledge the financial assistance provided by the Department of Science and Technology, Government of India for the present study under the Scheme WOS-A. The authors would also like to thank Central Instrumentation Facility, IISER Bhopal, India for providing NMR and Mass Spectra Analysis and Material Science Department of VNIT, Nagpur, India for FT-IR analysis respectively. We acknowledge our student, Deepak Khushalani for his help in acquiring the spectral data of KCR3A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bundale, S., Singh, J., Begde, D. et al. Rare actinobacteria: a potential source of bioactive polyketides and peptides. World J Microbiol Biotechnol 35, 92 (2019). https://doi.org/10.1007/s11274-019-2668-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2668-z