Abstract
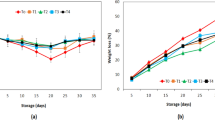
Cordyceps militaris is a model species of Cordyceps fungi, and has been traditionally used as an edible and medicinal fungus due to its richness of bioactive and pharmacological metabolites. The fruiting bodies of this fungus are widely used as healthy food and nutrition supply. In industrial production, fruiting bodies are cultivated on artificial media, but their yield and quality are usually affected by the quality of fungal strains. In this study, the effect of colony growth rate of the fungal strains, fungal age and repeated subculturing on the fungal biomass accumulation was investigated. The results indicated that the fungal biomass was positively correlated with the colony growth rate and not affected by fungal age and the repeated subculturing. The preservation conditions for stock cultures, including choice of cultures, lyophilization, temperature and protective agents were optimized based on the mycelial formation and conidia production in artificial inoculum. The development of fruiting bodies from the fungal strains stored under the optimized preservation conditions were further analyzed to determine the ideal time period of preservation. Results indicated that storing the fungus at 4 °C could maintain the fungal vitality and fruiting body producing capacity for at least 12 months. This study established practical criteria of fungal inoculum for artificial cultivation of fruiting body and provided a simple and efficient preservation method for C. militaris. The results may shed light on preservation for other Cordyceps species and other edible fungi.





Similar content being viewed by others
References
Ayala-Zermeño MA, Gallou A, Berlanga-Padilla AM, Andrade-Michel GY, Rodríguez-Rodríguez JC, Arredondo-Bernal HC, Montesinos-Matías R (2017) Viability, purity, and genetic stability of entomopathogenic fungi species using different preservation methods. Fungal Biol 121:920–928
Bainard LD, Klironomos JN, Hart MM (2010) Differential effect of sample preservation methods on plant and arbuscular mycorrhizal fungal DNA. J Microbiol Meth 82:124–130
Baskarathevan J, Jaspers MV, Jones EE, Ridgway HJ, Zydenbos SM (2009) Evaluation of different storage methods for rapid and cost-effective preservation of Botryosphaeria species. New Zealand Plant Protect 62:234–237
Clarkson JM, Charnley AK (1996) New insights into the mechanisms of fungal pathogenesis in insects. Trends Microbiol 4:197–203
Dahmen H, Staub T, Schwinn FJ (1983) Technique for long-term preservation of phytopathogenic fungi in liquid nitrogen. Phytopathology 73:241–246
De Freitas A, Araújo MGBA, Hendges EA et al (2014) Viability of Metarhizium anisopliae conidia (metsch.) Sorok preserved in packages containing silica gel. BMC Proc 8(S4):128
Dong CJ (2013) The traditional Chinese medicine fungus Cordyceps and its biotechnological production. Res J Biotechnol 8:1–2
Dong C, Liu X, Li Z et al (2016) Cordyceps industry in China: current status, challenges and perspectives –Jinhu declaration for cordyceps industry development. Mycosystema 35:1–15
Fang S, Lin Q, Ni ZJ (2011) Comparative study on the preservation methods of Cordyceps sinensis culture. Med Plant 2:13–16
Fukumoto F (2008) Preservation of alfalfa mosaic virus, by freezing and freeze-drying and similarities to Cucumoviruses. J Gen Plant Pathol 74:164–170
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Homolka L (2013) Methods of cryopreservation in fungi. Lab Protoc Fungal Biol 19:9–16
Homolka L (2014) Preservation of live cultures of basidiomycetes–recent methods. Fungal Biol 118:107–125
Ito T, Nakagiri A (1996) Viability of frozen cultures of Basidiomycetes after fifteen-year storage. Microbiol Cult Coll 12:67–78
Jong SC, Edwards MJ (1991) American type culture collection: Catalogue of filamentous fungi. American Type Culture Collection, Rockville
Karaduman AB (2012) An example for comparison of storage methods of macrofungus cultures: Schizophyllum commune. Turkish J Bot 36:205–212
Kitamoto Y, Suzuki A, Shimada S, Yamanaka K (2002) A new method for the preservation of fungus stock cultures by deep-freezing. Mycoscience 43:143–149
Lee HH, Lee S, Lee K, Yu SS, Kang H, Cho H (2015) Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. DARU J Pharm Sci 23:1–8
Li BQ, Tian SP (2007) Effect of intracellular trehalose in Cryptococcus laurentii and exogenous lyoprotectants on its viability and biocontrol efficacy on Penicillium expansum in apple fruit. Lett Appl Microbiol 44:437–442
Lin QY, Song B (2006) Optimization of some cultivation conditions of Cordyceps militaris. Edible Fungi China 25:17–19
Lin QQ, Qiu XH, Zheng ZL, Xie CH, Xu ZF, Han RC (2010) Characteristics of the degenerate strains of Cordyceps militaris. Mycosystema 29:670–677
Liu D, He LL, Wang ZQ, Chen JQ (2006) The influences of subculture of Cordyceps militaris to colonial morphology and fruit-body yield. J Shenyang Agricul Univer 37:538–541
Lomberh M, Buchalo A, Solomko E, Grygansky A, Kirchhoff B (2000) Investigation of mycelium growth and fruit body development of different strains of the beech mushroom Shimeji [Hypsizygus marmoreus (Bull.: Fries) Singer]. Science and Cultivation of Edible Fungi. In: Proceedings of 15th International Congress of the Science and Cultivation of Edible Fungi 2:763–770
López Lastra CC, Hajek AE, Humber RA (2002) Comparing methods of preservation for cultures of entomopathogenic fungi. Can J Bot 80:1126–1130
Pasanen AL, Kalliokoski P, Pasanen P (1991) Laboratory studies on the relationship between fungal growth and atmospheric temperature and humidity. Environ Int 17:225–228
Pietikäinen J, Pettersson M, Bååth E (2005) Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol Ecol 52:49–58
Prakash O, Nimonkar Y, Shouche YS (2013) Practice and prospects of microbial preservation. FEMS Microbiol Lett 339:1–9
Santoro PH, Zorzetti J, Constanski K, Neves PMOJ (2014) Conidial production, virulence, and stress tolerance of Beauveria bassiana conidia after successive in vitro subculturing. Rev Colomb Entomol 40:85–90
Shrestha B, Han SK, Lee WH, Choi SK, Lee J, Sung JM (2005) Distribution and in vitro fruiting of Cordyceps militaris in Korea. Mycobiology 33:178–181
Shrestha B, Zhang W, Zhang Y, Liu X (2012) The medicinal fungus Cordyceps militaris: research and development. Mycol Prog 11:599–614
Siddiqui ZA, Kataoka R (2011) Mycorrhizal inoculants: progress in inoculant production technology. Springer, New York
Sun SJ, Deng CH, Zhang LY, Hu KH (2017) Molecular analysis and biochemical characteristics of degenerated strains of Cordyceps militaris. Arch Microbiol 199:939–944
Sung GH, Hywel-Jones NL, Sung JM, Luangsa-ard JJ, Shrestha B, Spatafora JW (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57:5–59
Taniwaki MH, Pitt JI, Hocking AD, Fleet GH (2006) Comparison of hyphal length, ergosterol, mycelium dry weight, and colony diameter for quantifying growth of fungi from foods. Adv Exp Med Biol 571:49–67
Wang C, Butt TM, St Leger RJ (2005) Colony sectorization of Metarhizium anisopliae is a sign of ageing. Microbiology 151:3223–3236
Xia Y, Luo F, Shang Y, Chen P, Lu Y, Wang C (2017) Fungal cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin. Cell Chem Biol 24:1479–1489
Xu Q, Feng M (2001) Effect of plant oils on liquid culture and preservation of entomophthoralean fungi. Mycosystema 20:79–86
Zhang G, Liang Y (2013) Improvement of fruiting body production in Cordyceps militaris by molecular assessment. Arch Microbiol 195:579–585
Zhang Z, Shi JL (2010) Liquid cultivation of edible mushroom in oat emulsion and development of beverage from the culture. Food Sci 31:169–174
Zhang W, Cheng X, Liu X, Xiang M (2016) Genome studies on nematophagous and entomogenous fungi in China. J Fungi 2:9
Zhong S, Ji DF, Chen S, Hu GY, Li YG (2006) Research advancement of Cordyceps militaris. Bull Seric 37:6–10
Acknowledgements
This study was funded by Program for Liaoning Excellent Talents in University (LR2015058) and the Scientific Research Foundation for the Introduced Talents of Shenyang Agricultural University (20153040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11274_2018_2550_MOESM1_ESM.tif
Fig. 1 Mycelial growth determined by principal component analysis. Mycelial growth rate with the initial diameter of each biological replicate by linear regression model was further analyzed. Colors and shapes indicate five biological replicates with technical replicates, respectively. Supplementary material 1 (TIF 2074 KB)
11274_2018_2550_MOESM2_ESM.tif
Fig. 2 Culture index and sporulation of Cordyceps militaris preserved with liquid cultures. (a) Culture index of the inoculation cultures incubated from the fungal strains preserved with the lyophilization, temperatures [4 °C, room temperature (RT), − 20 °C, and − 80 °C], and protective agents ( 6% peptone, pasteurized skim milk, sterilized skim milk, and untreated). (b) Conidial abundance. Supplementary material 2 (TIF 3013 KB)
Rights and permissions
About this article
Cite this article
Sun, H., Hu, T., Guo, Y. et al. Preservation affects the vegetative growth and fruiting body production of Cordyceps militaris. World J Microbiol Biotechnol 34, 166 (2018). https://doi.org/10.1007/s11274-018-2550-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2550-4