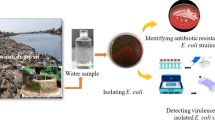
Abstract
The resistance of different pathogenic variants of E. coli to antibiotics, is a health concern globally. The study assessed the resistance of 90 E. coli isolates that survived chlorination at a Wastewater Treatment Plant (WWTP) in North West, South Africa (NW-SA), to 12 different antibiotics using the Kirby-Bauer disk diffusion method. The study further assessed the diarrheagenic pathotypes origin of the isolates. The molecular characterization revealed diarrheagenic E. coli pathotypes ranged as follows: Enteroaggregative E. coli (EAEC) 16 (17.78%), Enteroinvasive E. coli (EIEC) 6 (6.67%), Enterotoxigenic E. coli (ETEC) 5 (5.56%) and Enteropathogenic E. coli (EPEC) 3 (3.33%). A high degree of resistance was observed against sulphamethoxazol (92.22%), while lower resistance was observed against Kanamycin (3.33%), chloramphenicol (5.56%) and ciprofloxacin (6.67%). Multiple drug resistance of three and more antibiotics was observed in 81.11% of the E. coli isolates. The detected diarrheagenic E. coli pathotypes showed multiple resistance to different studied antibiotics with Multiple Antibiotic Resistance Indexing (MARI) equal to 0.9 for EIEC and EAEC respectively, followed by ETEC at 0.8 and EPEC at 0.2. The study reveals that the wastewater effluent from the studied plant serves as an important reservoir for the distribution of antibiotic resistant diarrheagenic E. coli pathotypes and other potential pathogens to the aquatic milieu, thus confirming potential risk to public health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Escherichia coli is a Gram-negative facultative anaerobe bacteria that has caught the attention of researchers since its discovery in 1885 (Tenaillon et al., 2010; Zhi et al., 2016). Escherichia coli presents a possible significant pathogenicity if released into the receiving water environment via inadequately treated wastewater (Osuolale & Okoh, 2017). It is for this reason that WWTP are important facilities for treatment of wastewater to avert environmental pollution that can pose significant risk to aquatic and public health (West & Mangiameli, 2000). Wastewater Treatment Plants have the responsibility to treat industrial and domestic wastewater discharge and ensuring that microbial load in effluents from the treatment plants are significantly reduced to meet the acceptable standard. In recent years, many treatment facilities have been reported to discharge effluents contaminated with relatively high level of pathogenic microorganisms (Anastasi et al., 2012; Makuwa et al., 2020), causing a possible risk to human health.
Some communities in SA still rely on polluted surface waters that are possibly contaminated by inadequately treated wastewater effluents for their domestic needs (Jagals, 1997; Omar & Barnard, 2010). The contamination of surface water with sewage waste can be monitored through the detection of E. coli and fecal coliform bacteria that serve as indicators of the presence of disease causing microorganisms (Tallon et al., 2005; Young & Thackston, 1999). Most strains of E. coli are harmless, but some are pathogenic and known to cause variety of gastrointestinal and extraintestinal disease (Kaper et al., 2004; Nataro & Kaper, 1998; Xia et al., 2011). To date, there are several studies that have reported the identification of intestinal pathogenic E. coli (IPEC) or diarrheagenic E. coli (DEC) groups from WWTP effluents. The pathotypes in this regard, include enteropathogenic E. coli (EPEC), enterohaemorrhagic/Shiga-toxin producing E. coli (EHEC)/STEC, enteroinvasive E. coli (EIEC), enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), diffusely adherent E. coli (DAEC) (Croxen & Finlay, 2010; Nataro & Kaper, 1998; Omar & Barnard, 2010; Robins-Browne et al., 2016; Shabana et al., 2013) and extraintestinal E. coli (ExPEC) (Köhler & Dobrindt, 2011; Russo & Johnson, 2000; Xia et al., 2011). Diarrheagenic E. coli is the main cause of worldwide epidemic and endemic diarrhea (Bonkoungou et al., 2012; Kaper et al., 2004; Shetty et al., 2012). Extraintestinal E. coli strains cause infections of any organ or anatomical site due to specialized virulence factors that are known to cause a broad spectrum of diseases and are not present on commensal E. coli (Kaper et al., 2004; Russo & Johnson, 2000).
Previous studies have shown an increase in antibiotic resistance bacteria from WWTPs effluents and that had recently led to a global concern (Dolejska et al., 2011; Redhead et al., 2020; Rizzo et al., 2013; Vaz-Moreira et al., 2014). Wastewater treatment facilities serve as a primary water reservoir and key potential gateways for antibiotic-resistant bacteria including E. coli’s of human and animal origin interfacing within the aquatic environment (Dolejska et al., 2011; Martinez, 2009; Osuolale & Okoh, 2017). The inadequate treatment of wastewater by treatment facilities often introduces pathogens and antibiotic-resistant E. coli into natural water resources (Rizzo et al., 2013; Vaz-Moreira et al., 2014), escalating the risk of infection (Dolejska et al., 2011; Igwaran et al., 2018; Ivanov et al., 2005; Martinez, 2009; Osuolale & Okoh, 2017). Bacterial populations received by wastewater treatment facilities from diverse sources interact and exchange antibiotic-resistant genes horizontally (Arana et al., 2001; Igwaran et al., 2018; Karkman et al., 2018). As an example, there are reports on Escherichia coli from WWTP effluents that have been shown to be resistant to several number of medically significant antibiotics (Abdul et al., 2013; Buvens et al., 2010; Osuolale & Okoh, 2017).
The treatment facility in this study was recently reported to discharging effluents contaminated with chlorine-disinfection resistant E. coli (Makuwa et al., 2020). The aim of the currently study is therefore, to assess the pathogenicity and antibiotic resistance profiles of the E. coli strains at the WWTP. Studying the antimicrobial resistance pattern of the pathogenic E. coli strains, is noteworthy in order to determine the shift in antibiotic resistance patterns among the pathogens and to adapt control measures that will help prevent the discharging of pathogenic multidrug-resistant E. coli strains to the environment.
2 Materials and Method
2.1 Study area
The plant of interest in this study (Coordinates: latitude: -26.75141 longitude: 27.0945) is situated in the North West Province of South Africa (NW-SA). The town where the plant is situated has a population of about 124,000 and it is an industrial and agricultural area for North West Province (Makuwa et al., 2020). The plant therefore receives municipal domestic sewage and wastewater that is heavily influenced by household and industrial water use. The plant is an activated sludge treatment plant. The treatment of physicochemical impurities is done through preliminary, primary, and secondary stages. The secondary stage operates through the Phoredox and Bardenpo activated sludge configurations.
There are different disinfection processes for treatment of wastewater in SA, of which chlorination is the most commonly applied (Bekink & Nozaic, 2013; Virto et al., 2005; Yang & Zhang, 2013). The plant studied uses chlorine gas as disinfectant. A dosing of 10 kg of chlorine/h is applied across all seasons. The studied plant uses chlorination as a form of disinfection. The contact time for disinfection at the tertiary treatment stage is 30 min.
2.2 Sample collections
A total of 90 WWTP final effluent samples were collected aseptically at the final discharge point using sterile 250 mL sampling bottles for the analysis of E. coli. The final discharge point is a point situated after disinfection process before treated wastewater enter the environment. The sampling containers were washed with soap and water and autoclaved after each use. Samples were collected on a weekly bases, between May 2019 and March 2020.
2.3 Isolation and confirmation of presumptive E. coli
The Colilert Quanti-Tray/2000 system as described in Omar et al., (2010) was used for the enumeration of the viable E. coli cells from the 90 samples studied. Enumeration of E. coli from samples was done by using 100 mL water according to the manufacturer’s instructions. The Quanti-Trays were incubated for 18–22 h at 37 ℃. After incubation, the Quanti-Trays/2000 were examined under long wave (366 nm) ultraviolet light, and wells that turned both yellow and fluoresced were counted as E. coli positive (IDDEX). The results of the quantifications were reported as E. coli count/100 mL. To get colonies for DNA extraction, isolates from the fluorescence wells of the Colilert Quanti-Tray/2000 system were sub-cultured on Eosin Methylene Blue agar (EMB agar) (Merck, Germany) and incubated at 37 °C for 24 h. Colonies of E. coli isolates were confirmed by a distinctive metallic green sheen appearance on EMB agar.
2.4 DNA extraction
Two colonies of pure isolated bacteria were placed into a tube containing 100 μL of double distilled water. The tubes were heated at 100 °C for 10 min, and then the cells were pelleted by centrifugation. The supernatant containing DNA was taken out and stored at -20 °C (Kazemnia et al., 2014; Obeng et al., 2012).
2.5 Molecular confirmation of the different diarrheagenic pathotypes
The confirmed isolates were delineated by using PCR into different E. coli diarrheagenic pathotypes based on the presence of virulence genes in their genome according to Tanih et al., (2015). The list of the studied diarrheagenic genes were categorised based on their functional characteristics (see Table 1). The diarrheagenic pathotypes of the confirmed E. coli isolates were determined with the aid of PCR technique using of specific primers targeting LT and ST genes for ETEC, stx1 and stx2 genes for EHEC, eae and bfpA genes for EPEC, ipaH gene for EIEC, aaTA and aaic genes for EAEC, as well as daaE gene for DAEC, as listed in Table 2.
Multiplex polymerase chain reaction analysis of the targeted genes of interest was performed using DreamTaq DNA polymerase (Thermo Scientific, USA). For the amplification, 5 μL of DNA was added to 20 μL of master mix containing 12.5 μL of DreamTaq DNA polymerase (2X DreamTaq Green Buffer, dATP, dCTP, dGTP, and dTTP, 0.4 mM each, and 4 mM MgCl2) (Thermo Scientific, USA), 0.5 μL (0.2 μM) of respective oligonucleotide primers. The reaction volume was made up with nuclease free water. PCR was performed in a thermal cycler (Bio-Rad Laboratories, USA). The reactions were subjected to an initial activation step at 95 °C for 15 min, followed by 35 cycles consisting of denaturing at 94 °C for 45 s, annealing at 55 °C for 45 s, extension at 68 °C for 2 min and final elongation at 72 °C for 5 min (Omar & Barnard, 2010). The primers used to amplify the targeted genes were as previously reported by Tanih et al., (2015) and Igwaran et al., (2018) as detailed in Table 2. Negative controls, substituting DNA template with ultrapure water (Sigma-Aldrich, UK), were included in all PCR runs. DNA extracted from E. coli ATCC 25922 was used as a positive control. Amplified DNA was resolved by 2% agarose gel electrophoresis and visualised under UV transillumination.
2.6 Antibiotic resistance testing
The resistance/susceptibility testing of all E. coli isolates was performed using the Kirby-Bauer disk diffusion method as described in the study by Kazemnia et al., (2014) and Osuolale and Okoh, (2017). A volume of 100 μL of an overnight growth E. coli isolate on Nutrient broth with 0.5 McFarland standard turbidity was streaked on Mueller- Hinton agar plates (Conda, Madrid). The study used 12 antibiotic discs, all from HiMedia® (India). The antibiotics included: cephazolin, gentamicin, ciprofloxacin, streptomycin, trimethoprim, amoxycillin, neomycin, kanamycin, chloramphenicol, sulphamethoxazol, nalidixic acid and tetracycline. The choices of antibiotic panels selected were based upon the recommendation of CLSI (CLSI, 2012). The group arrangements of these studied antibiotics were as follows: Cephems (cephazolin), Aminoglycosides (gentamicin, streptomycin, neomycin and kanamycin), Quinolones and Fluoroquinolones (ciprofloxacin and nalidixic acid), Folate Pathway Antagonists (trimethoprim and sulphamethoxazol), β-Lactam Combination Agent (amoxicillin), Phenicols (chloramphenicol), and Tetracyclines (tetracycline) (see Table 3). The antibiotics discs were placed on the surface of the inoculated Mueller-Hinton agar plates. After 10 min at room temperature, the plated cultures were incubated in an inverted position at 37 °C for 20–24 h. The zones of inhibition were measured and compared with standard chart.E. coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used as antibiotic controls. Isolates with intermediate resistance were defined as susceptible, and the isolates were considered as multidrug resistant if they were resistant to at least three classes of antibiotics (Bashir et al., 2011; Blanco et al., 2011; Bukh et al., 2009; Kazemnia et al., 2014).
2.7 Multiple Antibiotic Resistance Indexing (MARI)
Multiple antibiotic resistant (MAR) phenotypes were generated for strains that showed resistance to three or more antibiotics. MAR index was calculated as previously described by Osuolale, (2015) and is mathematically expressed as:
where
- a:
-
number of antibiotics to which the isolate was resistant;
- b:
-
total number of antibiotics against which individual isolate was tested.
3 Results
3.1 Molecular characterization of E. coli isolates
A total of 90 presumptive E. coli isolates were obtained from WWTP final effluent samples through Colilert Quanti-Tray/2000 (Omar et al., 2010). The 90 presumptive E. coli isolates recovered from the fluorescent Quanti-tray wells, were further confirmed by their distinctive metallic green sheen appearing on the surface of the bacterial colonies on EMB agar (Leininger et al., 2001). Among the 90 confirmed E. coli isolates assessed for the various diarrheagenic E. coli genes, 32 (35.56%) harbored at least 1 or more virulent genes while 58 (64.44%) isolates harbored none. The outcome of the different diarrheagenic pathotypes from E. coli isolates as indicated in Table 4, showed positive detection of ETEC 5 (5.56%), EPEC 3 (3.33%), EIEC 6 (6.67%), and EAEC 16 (17.78%) from the six studied diarrheagenic pathotypes, while no detections were observed for EHEC and DAEC E. coli pathotypes.
The diarrheagenic E. coli genes were categorised into toxin, adhesion and invasion genes based on functional characteristics of the genes as indicated in Table 1. Such categorisation enabled the study to identify the prevalence of these virulence genes with observable differences to each sample. The targeted genes were as follows: ETEC (LT and ST), EHEC (stx1 and stx2), EPEC (eae and bfpA), EAEC (aatA and aaic), EIEC (ipaH) and DAEC (daaE). The distribution of the targeted genes as presented in Table 4, showed aatA and aaiC genes dominating with 9 (10%) and 8 (8.89%) positive isolates, respectively. Other diarrheagenic pathotypes genes were variously detected as follows; ipaH 6 (6.67%), ST 5 (5.56%), eae 3 (3.33%) and LT 1 (1.11%). Of the 5 positive ETEC isolates shown in Table 4, 1 (20%) isolate presented both of ST and LT genes, while similar results of single isolates (6.25%) were also observed with genes (aatA and aaic) meant to confirm the presence of EAEC among the 16 detected isolates. Both EHEC and EPEC did not show shared genes amongst their isolates, while EIEC and DAEC isolates were detected using single genes primers. The representative gel electrophoresis profiles of amplified products of the investigated diarrheagenic E. coli virulence genes are shown in Fig. 1.
A representative gel electrophoresis profile of different virulence genes of isolated E. coli. Lane 1: molecular weight marker (Merck 1 kb DNA ladder), lane 2: negative control, lane 3: LT (508 bp), lane 4: LT (508 bp), lane 5: negative test, lane 6: negative test, lane 7: aatA (650 bp), lane 8: aatA (650 bp), lane 9: aatA (650 bp), lane 10: negative test, lane 11: eae (881 bp), lane 12: negative test, lane 13: eae (881 bp), lane 14: eae (881 bp), lane 15: eae (881 bp), lane 16: eae (881 bp), lane 17: ipaH (423 bp), lane 18: ipaH (423 bp), lane 19: aatA (650 bp), lane 20: negative control
3.2 Antibiotic resistance profiling of confirmed E. coli isolates
A total of 12 antibiotics were assessed against the 90 E. coli isolates extracted from WWTP final effluent samples. The presence of E. coli in the final effluent confirmed their survival to chlorination which is the disinfection method applied at the studied plant. The antibiotics included: cephazolin, gentamicin, ciprofloxacin, streptomycin, trimethoprim, amoxicillin, neomycin, kanamycin, chloramphenicol, sulphamethoxazol, nalidixic acid and tetracycline.
The antibiotic susceptibility, intermediate and resistant profiles of the E. coli isolates are presented in Fig. 2. The E. coli isolates were mostly resistant to sulphamethoxazol, with less resistance observed against Kanamycin (3.33%), chloramphenicol (5.56%) and ciprofloxacin (6.67%). The overall resistance profiles of the E. coli isolates were as follows: sulphamethoxazol (92.22%), tetracycline (56.67%), trimethoprim (52.22%), neomycin (48.89%), nalidixic acid (41.11%) streptomycin (40%), amoxicillin (40%), cephazolin (37.78%), gentamicin (12.22%), ciprofloxacin (6.67%), chloramphenicol (5.56%) and kanamycin (3.33%). Multi-antibiotic resistance was considered when the isolate was resistant to three and more antibiotics with 81.11% of multi drug resistance cases observed.
3.3 Antibiotic resistance profiling of confirmed diarrheagenic E. coli pathotypes
The antibiotic profiles of the studied diarrheagenic E. coli pathotypes are shown in Table 5. Both EIEC and EAEC showed multiple resistance to all the studied antibiotics, except for Kanamycin (EIEC) and Chloramphenicol (EAEC) respectively. The least resistance was observed in EAEC with regard to Kanamycin (6%). EIEC (0.9), EAEC (0.9) and ETEC (0.8) showed highest MAR index in relation to antiobiotic resistance, while EPEC (0.2) showed the least MAR index.
4 Discussion
Escherichia coli is commonly known as an indicator that predict possible presence of other pathogens of enteric origins (Cabral, 2010; Jamieson et al., 2002; Motlagh & Yang, 2019; Rompré et al., 2002). This organism underlines the importance of municipal WWTPs as potential point sources of pathogens into environmental waters (Adefisoye & Okoh, 2016). In this study, the prevalence, and the antibiotic resistance profiling of diarrheagenic E. coli remoted from NW-SA WWTP final effluent samples, were investigated. Diarrheagenic E. coli are primary etiological agents of pediatric diarrhea, which remains the most common cause of infantile morbidity and mortality especially in developing countries. The organisms are transmitted through the oral-fecal path by ingesting food or water contaminated by human or animal feces (Adefisoye & Okoh, 2016).
Diarrheagenic E. coli are the principal cause of demise globally, especially in developing nations (Bryce et al., 2005; Igwaran et al., 2018; Shabana et al., 2013). Amongst the six diarrheagenic E. coli pathotypes profiled from the 90 confirmed E. coli isolates, only ETEC, EPEC, EAEC, and EIEC were detected, while none of the isolates showed targeted virulence genes for EHEC and DAEC. These pathotypes were identified based on the targeted genes shown in Tables 1 and 2, however contrary to the study by Igwaran et al., (2018), daaE gene for DAEC was not identified in this study. The presence of these diarrheagenic E. coli pathotypes in the environment calls for concern due to their public health concerns (Clements et al., 2012; Haller et al., 2009). The detected diarrheagenic E. coli pathotypes implies that the studied WWTP serves as a reservoir of the diarrheagenic E. coli pathotypes. Studies by Adefisoye and Okoh, (2016) and Omar and Barnard, (2010) in EC-SA and Gauteng-SA respectively, detected the presence of diarrheagenic E. coli from the final effluent of wastewater treatment plant and this also substantiated our findings. Of the 90 confirmed E. coli isolates tested, EAEC represented about 17.78% of isolates, followed by EIEC at 6.67%, ETEC at 5.56% and EPEC at 3.33%. In similar studies by Mbanga et al., (2020) and Omar and Barnard, (2010), they reported a high detection of EAEC in their treated final effluents samples. In the Eastern Cape and Limpopo Provinces of South Africa, these diarrheagenic E. coli pathotypes have been isolated from diarrhea patients, with EAEC being the predominant cause of infection (Bisi-Johnson et al., 2011; Samie et al., 2007). A single isolate each for ETEC and EAEC showed the presence of both targeted genes. Similar observation of shared genes (LT and ST) for ETEC isolate, were observed in previous studies by Bolukaoto et al., (2021) and Hossain et al., (2021). According to Johura et al. (2017), the genes that encode both LT and ST enterotoxins in ETEC are generally found on plasmids, transmissible and causing severe diarrhea. The EAEC gene coding for both aaic and aatA, were also observed at a higher rate than this study from children in Norh-eastern Brazil (Lima et al., 2013).
Antimicrobial resistance testing is a famous global standard allowing laboratories to help clinicians in treating infections caused by microbial agents (Igwaran et al., 2018; Rizzo et al., 2013). The prevalence of antimicrobial resistant bacteria in WWTP effluents is a foremost public health concern (Mukherjee et al., 2021; Rodriguez-Molina et al., 2019). Effluents from WWTP used for irrigation water for crops can stimulate the distribution of antibiotic resistance genes into soils and could by some means find their ways into human system (Wang et al., 2014). The final effluent of WWTPs has been identified by studies as a prime vehicle of antibiotics resistant pathogens into the aquatic environment (Igwaran et al., 2018; Osunmakinde et al., 2019; Zerva et al., 2021).
From the confirmed E. coli isolates that were tested against a panel of 12 commercial antibiotics, the isolates divulged distinct resistance patterns against the antibiotics. The E. coli isolated from the discharged effluent, survived chlorine disinfection. With the E. coli isolates having survived chlorine disinfection, the antibiotic-resistant bacteria also demonstrated the capability for re-growth to a chlorine disinfection of up to 5 mg/L in a study by Destiani and Templeton, (2019). According to Huang et al., (2011), some antibiotic-resistant bacteria have been reported to demonstrate resistance to chlorine. Sulphamethoxazole and tetracycline was the highest-level resistance antibiotic, this finding corroborate the results from a study by Osuolale, (2015). Osuolale, (2015), associated the high resistance level of sulphamethoxazole to domestic, industrial and health facility wastes, surface runoff and various anthropogenic activities.
Antibiotic resistance, particularly multidrug resistance, is a major public health threat and is an emerging concern around the world (Watkinson et al., 2007). About 81.11% of the isolates showed resistance to more than three antibiotics. Adefisoye and Okoh, (2016) observed lower multidrug of 32.7% compared to this study. Multidrug resistance of E coli isolates was observed in 85.11% of the hospital wastewater and 73.53% of the community wastewater studied by Gașpar et al., (2021). With E. coli being the most commonly studied bacteria, the resistance to at least two classes of antimicrobial agents has been regularly detected in the environment (Baum & Marre, 2005; Young, 1993). The current study revealed multidrug resistance to numbers of antibiotics ranging from 3 to 10, while Osuolale, (2015) reported multidrug ranging between 3 to 9 antibiotics. The antibiotic resistance patterns of the E. coli isolates were as follows: no-antibiotic resistance (0), single-antibiotic resistance (1), two-antibiotic resistance (7), three-antibiotic resistance (11), four-antibiotic resistance (13), five-antibiotic resistance (15), six-antibiotic resistance (13), seven-antibiotic resistance (18), eight-antibiotic resistance (8), nine-antibiotic resistance (3) and ten-antibiotic resistance (1). The antibiotic resistance patterns in the study by Osuolale, (2015) was as follows: no-antibiotic resistance (4), single-antibiotic resistance (36), two-antibiotic resistance (25), three-antibiotic resistance (19), four-antibiotic resistance (21), five-antibiotic resistance (24), six-antibiotic resistance (20), seven-antibiotic resistance (6), eight-antibiotic resistance (13) and nine-antibiotic resistance (5). According to the study by Murray et al., (1984), wastewater effluent disinfection has also been shown to increase the prevalence of antibiotic resistant bacteria and multidrug resistance.
Infections triggered by diarrheagenic E. coli pathotypes are treated with antibiotics; however, the emergence of resistant strains may affect the treatment of some infections (Ishii & Sadowsky, 2008). All the detected diarrheagenic E. coli pathotypes presented higher level of resistance to Sulphamethoxazol. Study by Osuolale and Okoh, (2017), showed higher resistance of isolated pathogens to tetracycline. EPEC and EIEC showed total resistance to Sulphamethoxazol. EIEC was the only diarrheagenic E. coli pathotype that completely showed resistance to Tetracyline. A study by Osuolale, (2015), revealed that, all their detected pathotypes showed total resistance to sulphamethoxazole and display a significant high resistance to ampicillin, amoxycillin, gentamycin, cefuroxin, tetracycline and chloramphenicol. In the study by Torres, (2009), EPEC was the common pathotype associated with multiple antibiotic resistances, while in other studies they were EPEC and ETEC (Oliveira et al., 2012), EAEC and EPEC (Hamelin et al., 2006). This study used MARI to estimate health threat related to the spread of antibiotic resistance in the environment. According to MARI calculation, the EIEC and EAEC showed highest multiple antibiotic resistance of 0.9, each, respectively, followed by ETEC (0.8) and EPEC (0.2). According to Christopher et al., (2013), MARI above 0.2 suggests that a strain(s) of bacteria originate from an environment with excessive contamination or antibiotics usage. The high MARI values observed with EIEC, EAEC and ETEC diarrheagenic E. coli pathotypes acquired in this study may advocate the exposure of the pathotypes to antibiotics pressure, which may have resulted from wrong use of antibiotic among the populace of the studied area and may lead to further increase in the development of multidrug resistance if proper processes are not applied. The resistance of the diarrheagenic E. coli pathotypes to studied antibiotics serves as a pointer to the possible presence of other E. coli pathotypes inclusive of other bacterial pathogens presenting resistance to several antibiotics. The findings of this study is in line with other reports on the detection of more than one antibiotic resistance through commensal and pathogenic strains of E. coli (Bailey et al., 2010; Karczmarczyk et al., 2011).
5 Conclusion
The mandate of a WWTP is to notably reduce microbial constituency before the plant effluent is discharged into the environment, however vast quantities of pathogenic antibiotic resistant bacteria escape the treatment process into aquatic milieu. The confirmation of the presence of E. coli from WWTP final effluent in NW-SA, indicates fecal contamination and the feasible presence of other enteric pathogens inclusive of different E. coli pathotypes. Our findings suggest an excessive prevalence of antimicrobial resistance of diarrheagenic E. coli pathotypes towards the conventionally used antibiotics, thus a presenting public health risk. It is therefore significant for the regulators to review their handling of wastewater and antibiotics wastes to lessen their environmental impacts, and public health concerns.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abdul, R. S. V. A., Suchitra, S. M., Taruna, Y., & Radhakrishna, M. (2013). The antibiotic susceptibility patterns of uropathogenic Escherichia coli, with special reference to the fluoroquinolones. Journal of Clinical and Diagnostic Research, 7(6), 1027–1030. https://doi.org/10.7860/JCDR/2013/4917.3038
Adefisoye, M. A., & Okoh, A. I. (2016). Identification and antimicrobial resistance prevalence of pathogenic Escherichia coli strains from treated wastewater effluents in Eastern Cape, South Africa. Microbiology Open, 5(1), 143–151. https://doi.org/10.1002/mbo3.319
Anastasi, E. M., Matthews, B., Stratton, H. M., & Katouli, M. (2012). Pathogenic Escherichia coli found in sewage treatment plants and environmental waters. Applied and Environmental Microbiology, 78(16), 5536–5541. https://doi.org/10.1128/AEM.00657-12
Arana, I., Justo, J. I., Muela, A., & Barcina, I. (2001). Survival and plasmid transfer ability of Escherichia coli in wastewater. Water, Air, and Soil Pollution, 126, 223–238.
Bailey, J. K., Pinyon, J. L., Anantham, S., & Hall, R. M. (2010). Commensal Escherichia coli of healthy humans: A reservoir for antibiotic-resistance determinants. Journal of Medical Microbiology, 59(Pt 11), 1331–1339. https://doi.org/10.1099/jmm.0.022475-0
Bashir, S., Sarwar, Y., Ali, A., Mohsin, M., Saeed, M. A., Tariq, A., & Haque, A. (2011). Multiple drug resistance patterns in various phylogenetic groups of uropathogenic E. coli isolated from Faisalabad region of Pakistan, Braz. Journal of Microbiology, 42(4), 1278–1283. https://doi.org/10.1590/S1517-83822011000400005
Baum, H. V., & Marre, R. (2005). Antimicrobial resistance of Escherichia coli and therapeutic implications. International Journal of Medical Microbiology, 295(6–7), 503–511. https://doi.org/10.1016/j.ijmm.2005.07.002
Bekink, M. J., & Nozaic, D. J. (2013). Assessment of a chlorine dioxide proprietary product for water and wastewater disinfection. Water SA, 39(3), 375–378. https://doi.org/10.4314/wsa.v39i3.5
Bisi-Johnson, M. A., Obi, C. L., Vasaikar, S. D., Baba, K. A., & Hattori, T. (2011). Molecular basis of virulence in clinical isolates of Escherichia coli and Salmonella species from a tertiary hospital in the Eastern Cape, South Africa. Gut Pathogens, 3(9), 1–8. https://doi.org/10.1186/1757-4749-3-9
Blanco, J., Mora, A., Mamani, R., López, C., Blanco, M., Dahbi, G., Herrera, A., Blanco, J. E., Alonso, M. P., García-Garrote, F., Chaves, F., Orellana, M. A., Martínez-Martínez, L., Calvo, J., Prats, G., Larrosa, M. N., lez-López, J. J. G., López-Cerero, L., Rodríguez-Baño, J., & Pascual, A. (2011). National survey of Escherichia coli causing extraintestinal infections reveals the spread of drug-resistant clonal groups O25b:H4–B2-ST131, O15:H1-D-ST393 and CGA-D-ST69 with high virulence gene content in Spain. Journal of Antimicrobial Chemotherapy, 66(9), 2011–2021. https://doi.org/10.1093/jac/dkr235
Bolukaoto, J. Y., Singh, A., Alfinete, N., & Barnard, T. G. (2021). Occurrence of Hybrid Diarrhoeagenic Escherichia coli Associated with Multidrug Resistance in Environmental Water, Johannesburg, South Africa. Microorganisms, 9(10), 2163. https://doi.org/10.3390/microorganisms9102163
Bonkoungou, I. J. O., Lienemann, T., Martikainen, O., Dembelé, R., Sanou, I., Traoré, A. S., Siitonen, A., Barro, N., & Haukka, K. (2012). Diarrhoeagenic Escherichia coli detected by 16-plex PCR in children with and without diarrhoea in Burkina Faso. Clinical Microbiology and Infection, 18(9), 901–906. https://doi.org/10.1111/j.1469-0691.2011.03675.x
Bryce, J., Boschi-Pinto, C., Shibuya, K., Black, R. E., WHO Child Health Epidemiology Reference Group. (2005). WHO estimates of the causes of death in children. Lancet, 365(9465), 1147–1152. https://doi.org/10.1016/S0140-6736(05)71877-8
Bukh, A. S., Schønheyder, H. C., Emmersen, J. M. G., Søgaard, M., Bastholm, S., & Roslev, P. (2009). Escherichia coli phylogenetic groups are associated with site of infection and level of antibiotic resistance in community-acquired bacteraemia: A 10 year population-based study in Denmark. Journal of Antimicrobial Chemotherapy, 64(1), 163–168. https://doi.org/10.1093/jac/dkp156
Buvens, G., Bogaerts, P., Glupczynski, Y., Lauwers, S., & Piérard, D. (2010). Antimicrobial resistance testing of verocytotoxin-producing Escherichia coli and first description of TEM-52 extended-spectrum β-lactamase in serogroup O26. Antimicrobial Agents and Chemotherapy, 54(11), 4907–4909. https://doi.org/10.1128/AAC.00551-10
Cabral, J. P. S. (2010). Water microbiology. Bacterial pathogens and water. International Journal of Environmental Research and Public Health, 7(10), 3657–3703. https://doi.org/10.3390/ijerph7103657
Christopher, A. F., Hora, S., & Ali, Z. (2013). Investigation of plasmid profile antibiotic susceptibility pattern multiple antibiotic resistance index calculation of Escherichia coli isolates obtained from different human clinical specimens at tertiary care hospital in Bareilly-India. Annals of Tropical Medicine and Public Health, 6(3), 285–289. https://doi.org/10.4103/1755-6783.120985
Clements, A., Young, J., Constantinou, N., & Frankel, G. (2012). Infection strategies of enteric pathogenic E. coli. Gut Microbes, 3(2), 0–16. https://doi.org/10.4161/gmic.19182
Clinical and Laboratory Standards Institute. (2012). Performance standards for antimicrobial susceptibility testing; Twenty-second informational supplement, CLSI. Approved standard—Eleventh edition. CLSI Document M02-A11. Clinical and Laboratory Standards Institute, Wayne, 32(1).
Croxen, M. A., & Finlay, B. B. (2010). Molecular mechanisms of Escherichia coli pathogenicity. Nature Reviews Microbiology, 8, 26–38. https://doi.org/10.1038/nrmicro2265
Destiani, R., & Templeton, M. R. (2019). Chlorination and ultraviolet disinfection of antibiotic-resistant bacteria and antibiotic resistance genes in drinking water. AIMS Environmental Science, 6(3), 222–241. https://doi.org/10.3934/environsci.2019.3.222
Dolejska, M., Frolkova, P., Florek, M., Jamborova, I., Purgertova, M., Kutilova, I., Cizek, A., Guenther, S., & Literak, I. (2011). CTX-M-15-producing Escherichia coli clone B2–O25b-ST131 and Klebsiella spp. isolates in municipal wastewater treatment plant effluents. Journal of Antimicrobial Chemotherapy, 66(12), 2784–2790. https://doi.org/10.1093/jac/dkr363
Gașpar, C. M., Cziszter, L. T., Lăzărescu, C. F., Tibru, L., Pentea, M., & Butnariu, M. (2021). Antibiotic Resistance among Escherichia coli Isolates from Hospital Wastewater Compared to Community Wastewater. Water, 13(23), 3449. https://doi.org/10.3390/w13233449
Haller, L., Amedegnato, E., Poté, J., & Wildi, W. (2009). Influence of freshwater sediment characteristics on persistence of fecal indicator bacteria. Water, Air, & Soil Pollution, 203(1–4), 217–227. https://doi.org/10.1007/s11270-009-0005-0
Hamelin, K., Bruant, G., El-Shaarawi, A., Hill, S., Edge, T. A., & Bekal, S. (2006). A virulence and antimicrobial resistance DNA microarray detects a high frequency of virulence genes in Escherichia coli isolates from Great Lakes recreational waters. Applied and Environmental Microbiology, 72(6), 4200–4206. https://doi.org/10.1128/AEM.00137-06
Hossain, Md.S., Ali, S., Hossain, M., Uddin, S.Z., Moniruzzaman, M., Islam, M.R., Shohael, A.M., Islam, Md,S., Ananya, T.H., Rahman, Md.M., Rahman,M.A., Worth, M., Mondal, D., & Mahmud, Z.H. (2021). ESBL Producing Escherichia coli in Faecal Sludge Treatment Plants: An Invisible Threat to Public Health in Rohingya Camps, Cox's Bazar, Bangladesh. Frontiers in Public Health, 9, 783019. https://doi.org/10.3389/fpubh.2021.783019
Huang, J. J., Hu, H. Y., Tang, F., Li, Y., Lu, S. Q., & Lu, Y. (2011). Inactivation and reactivation of antibiotic-resistant bacteria by chlorination in secondary effluents of a municipal wastewater treatment plant. Water Research, 45(9), 2775–2781. https://doi.org/10.1016/j.watres.2011.02.026
Igwaran, A., Iweriebor, B. C., & Okoh, A. I. (2018). Molecular characterization and antimicrobial resistance pattern of Escherichia coli recovered from wastewater treatment plants in Eastern Cape South Africa. International Journal of Environmental Research and Public Health, 15(6), 1237. https://doi.org/10.3390/ijerph15061237
Ishii, S., & Sadowsky, M. J. (2008). Escherichia coli in the environment: Implications for water quality and human health. Journal of Applied Microbiology, 23(2), 101–108. https://doi.org/10.1264/jsme2.23.101
Ivanov, V., Stabnikov, V., Zhuang, W. Q., Tay, J. H., & Tay, S. T. L. (2005). Phosphate removal from the returned liquor of municipal wastewater treatment plant using iron-reducing bacteria. Journal of Applied Microbiology, 98(5), 1152–1161. https://doi.org/10.1111/j.1365-2672.2005.02567.x
Jagals, P. (1997). Stormwater runoff from typical developed and developing South African urban developments: Definitely not for swimming. Water Science and TechnolOgy, 35(11–12), 133–140. https://doi.org/10.1016/S0273-1223(97)00248-5
Jamieson, R. C., Gordon, R. J., Sharples, K. E., Stratton, G. W., & Madani, A. (2002). Movement and persistence of fecal bacteria in agricultural soils and subsurface drainage water: A review. Canadian Biosystems Engineering / Le Genie des biosystems au Canada, 44, 1.1–1.9.
Johura, F. T., Parveen, R., Islam, A., Sadique, A., Rahim, M. N., Monira, S., Khan, A. R., Ahsan, S., Ohnishi, M., Watanabe, H., Chakraborty, S., George, C. M., Cravioto, A., Navarro, A., Hasan, B., & Alam, M. (2017). Occurrence of hybrid Escherichia coli strains carrying Shiga toxin and heat-stable toxin in livestock of Bangladesh. Frontiers in Public Health, 4, 287. https://doi.org/10.3389/fpubh.2016.00287
Kaper, J. B., Nataro, J. P., & Mobley, H. L. (2004). Pathogenic Escherichia coli. Nature Reviews Microbiology, 2, 123–140. https://doi.org/10.1038/nrmicro818
Karczmarczyk, M., Abbott, Y., Walsh, C., Leonard, N., & Fanning, S. (2011). Characterization of multidrug-resistant Escherichia coli isolates from animals presenting at a university veterinary hospital. Applied and Environmental Microbiology, 77(20), 7104–7112. https://doi.org/10.1128/AEM.00599-11
Karkman, A., Do, T. T., Walsh, F., & Virta, M. P. J. (2018). Antibiotic-Resistance Genes in Wastewater. Trends. Microbiology, 26(3), 220–228. https://doi.org/10.1016/j.tim.2017.09.005
Kazemnia, A., Ahmadi, M., & Dilmaghani, M. (2014). Antibiotic resistance pattern of different Escherichia coli phylogenetic groups isolated from human urinary tract infection and avian colibacillosis. Iranian Biomedical Journal, 18(4), 219–224. https://doi.org/10.6091/ibj.1394.2014
Köhler, C. D., & Dobrindt, U. (2011). What defines extraintestinal pathogenic Escherichia coli? International Journal of Medical Microbiology, 301(8), 642–647. https://doi.org/10.1016/j.ijmm.2011.09.006
Leininger, D. J., Roberson, J. R., & Elvinger, F. (2001). Use of eosin methylene blue agar to differentiate Escherichia coli from other gram-negative mastitis pathogens. Journal of Veterinary Diagnostic Investigation, 13(13), 273–275. https://doi.org/10.1177/104063870101300319
Lima, I. F. N., Boisen, N., Quetz, J. S., Havt, A., Carvalho, E. B., & Soares, A. M. (2013). Prevalence of enteroaggregative Escherichia coli and its virulence-related genes in a case-control study among children from north-eastern Brazil. Journal of Medical Microbiology, 62(Pt 5), 683–693. https://doi.org/10.1099/jmm.0.054262-0
Makuwa, S., Tlou, M., Fosso-Kankeu, E., & Green, E. (2020). Evaluation of fecal coliform prevalence and physicochemical indicators in the effluent from a wastewater treatment plant in the north-west province, South Africa. International Journal of Environmental Research and Public Health, 17(17), 6381. https://doi.org/10.3390/ijerph17176381
Martinez, J. L. (2009). Environmental pollution by antibiotics and by antibiotic resistance determinants. Environmental Pollution, 157(11), 2893–2902. https://doi.org/10.1016/j.envpol.2009.05.051
Mbanga, J., Abia, A. L., Amoako, D. G., & Essack, S. Y. (2020). Quantitative microbial risk assessment for waterborne pathogens in a wastewater treatment plant and its receiving surface water body. BMC Microbiology, 20(1), 1–12. https://doi.org/10.1186/s12866-020-02036-7
Motlagh, A. M., & Yang, Z. (2019). Detection and occurrence of indicator organisms and pathogens. Water Environment Research, 91(10), 1402–1408. https://doi.org/10.1002/wer.1238
Mukherjee, M., Laird, E., Gentry, T. J., Brooks, J. P., & Karthikeyan, R. (2021). Increased Antimicrobial and Multidrug Resistance Downstream of Wastewater Treatment Plants in an Urban Watershed. Frontiers in Microbiology, 12, 657353. https://doi.org/10.3389/fmicb.2021.657353
Murray, G. E., Tobin, R. S., Junkins, B., & Kushner, D. J. (1984). Effect of chlorination on antibiotic resistance profiles of sewage-related bacteria. Applied and Environmental Microbiology, 48(1), 73–77. https://doi.org/10.1128/aem.48.1.73-77.1984
Nataro, J. P., & Kaper, J. B. (1998). Diarrheagenic Escherichia coli. Clinical Microbiology RevIews, 11(1), 142–201. https://doi.org/10.1128/CMR.11.1.142
Obeng, A. S., Rickard, H., Ndi, O., Sexton, M., & Barton, M. (2012). Antibiotic resistance, phylogenetic grouping and virulence potential of Escherichia coli isolated from the faeces of intensively farmed and free range poultry. Veterinary Microbiology, 154(3–5), 305–315. https://doi.org/10.1016/j.vetmic.2011.07.010
Oliveira, K. W., Gomes, F. C. O., Benko, G., Pimenta, R. S., Magalhães, P. P., Mendes, E. N., & Morais, P. B. (2012). Antimicrobial resistance profiles of diarrhoeagenic Escherichia coli strains isolated from bathing waters of the Lajeado reservoir in Tocantins. Revista Ambiente and Água, 7(2), 30–41. https://doi.org/10.4136/ambi-agua.756
Omar, K. B., & Barnard, T. G. (2010). The occurrence of pathogenic Escherichia coli in South African wastewater treatment plants as detected by multiplex PCR. Water SA, 36(2), 172–176.
Omar, K. B., Potgieter, N., & Barnard, T. G. (2010). Development of a rapid screening method for the detection of pathogenic Escherichia coli using a combination of Colilert® Quanti-Trays/2000 and PCR. Water Science and Technology: Water Supply, 10(1), 7–13. https://doi.org/10.2166/ws.2010.862
Osunmakinde, C. O., Selvarajan, R., Mamba, B. B., & Msagati, T. A. M. (2019). Profiling Bacterial Diversity and Potential Pathogens in Wastewater Treatment Plants Using High-Throughput Sequencing Analysis. Microorganisms, 7(11), 1–18. https://doi.org/10.3390/microorganisms7110506
Osuolale, O., & Okoh, A. (2017). Detection and antibiotic susceptibility of pathogenic Escherichia coli isolated from the final effluent of two wastewater treatment plants in the Eastern Cape Province, South Africa. bioRxiv. https://doi.org/10.1101/160697
Osuolale, Y. T. (2015). Assessment of the quality indices and prevalence of Escherichia coli pathotypes in selected rivers of Osun state, PhD Thesis, University of Fort Hare, South Africa.
Redhead, S., Nieuwland, J., Esteves, S., Lee, D. H., Kim, D. W., Mathias, J., Cha, C. J., Toleman, M., Dinsdale, R., Guwy, A., & Hayhurst, E. (2020). Fate of antibiotic resistant E. coli and antibiotic resistance genes during full scale conventional and advanced anaerobic digestion of sewage sludge. PLoS ONE, 15(12), 1–17. https://doi.org/10.1371/journal.pone.0237283.eCollection
Rizzo, L., Manaia, C., Merlin, C., Schwartz, T., Dagot, C., Ploy, M. C., Michael, I., & Fatta-Kassinos, D. (2013). Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Science of the Total Environment, 447, 345–360. https://doi.org/10.1016/j.scitotenv.2013.01.032
Robins-Browne, R. M., Holt, K. E., Ingle, D. J., Hocking, D. M., Yang, J., & Tauschek, M. (2016). Are Escherichia coli pathotypes still relevant in the era of whole-genome sequencing? Frontiers in Cellular and Infection Microbiology, 6(141), 141. https://doi.org/10.3389/fcimb.2016.00141
Rodriguez-Molina, D., Mang, P., Schmitt, H., Chifiriuc, M. C., Radon, K., & Wengenroth, L. (2019). Do wastewater treatment plants increase antibiotic resistant bacteria or genes in the environment? Protocol for a systematic review. Systematic Reviews, 8(1), 304. https://doi.org/10.1186/s13643-019-1236-9
Rompré, A., Servais, P., Baudart, J., de-Robine, M. R., & Laurent, P. (2002). Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. Journal of Microbiological Methods, 49(1), 31–54. https://doi.org/10.1016/s0167-7012(01)00351-7
Russo, T. A., & Johnson, J. R. (2000). Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. Journal of Infectious Diseases, 181(5), 1753–1754. https://doi.org/10.1086/315418
Samie, A., Obi, C., & Dillingham, R. (2007). Enteroaggregative Escherichia coli in Venda, South Africa: Distribution of Virulence-Related Genes by Multiplex Polymerase Chain Reaction in Stool Samples of Human Immunodeficiency Virus (HIV)-positive and HIV-negative individuals and primary school children. American Journal of Tropical Medicine and Hygiene, 77(1), 142–150.
Shabana, I. I., Zaraket, H., & Suzuki, H. (2013). Molecular studies on diarrhea-associated Escherichia coli isolated from humans and animals in Egypt. Veterinary Microbiology, 167(3–4), 532–539. https://doi.org/10.1016/j.vetmic.2013.08.014
Shetty, V. A., Kumar, S. H., Shetty, A. K., Karunasagar, I., & Karunasagar, I. (2012). Prevalence and Characterization of Diarrheagenic Escherichia coli Isolated from Adults and Children in Mangalore. India. Journal of Laboratory Physicians, 4(1), 024–029. https://doi.org/10.4103/0974-2727.98666
Tallon, P., Magajna, B., Lofranco, C., & Leung, K. T. (2005). Microbial indicators of faecal contamination in water: A current perspective. Water, Air, and Soil Pollution, 166(1–4), 139–166.
Tanih, N. F., Sekwadi, E., Ndip, R. N., & Bessong, P. O. (2015). Detection of pathogenic Escherichia coli and Staphylococcus aureus from cattle and pigs slaughtered in abattoirs in Vhembe District, South Africa. Science World Journal, 2015(2015), 195972. https://doi.org/10.1155/2015/195972
Tenaillon, O., Skurnik, D., Picard, B., & Denamur, E. (2010). The population genetics of commensal Escherichia coli. Nature Reviews Microbiology, 8(3), 207–217. https://doi.org/10.1038/nrmicro2298
Torres, A. G. (2009). Intestinal pathogenic Escherichia coli. Vaccines for biodefence and emerging and neglected diseases, Elsevier Inc., pp1013–29. https://doi.org/10.1016/B978-0-12-369408-9.00051-2
Vaz-Moreira, I., Nunes, O. C., & Manaia, C. M. (2014). Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiology Reviews, 38(4), 761–778. https://doi.org/10.1111/1574-6976.12062
Virto, R., Mañas, P., Álvarez, I., Condon, S., & Raso, J. (2005). Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Applied and Environmental Microbiology, 71(9), 5022–5028. https://doi.org/10.1128/AEM.71.9.5022-5028.2005
Wang, F. H., Qiao, M., Lv, Z. E., Guo, G. X., Jia, Y., Su, Y. H., & Zhu, Y. G. (2014). Impact of reclaimed water irrigation on antibiotic resistance in public parks, Beijing, China. Environmental Pollution, 184, 247–253. https://doi.org/10.1016/j.envpol.2013.08.038
Watkinson, A. J., Murby, E. J., & Costanzo, S. D. (2007). Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Research, 41(18), 4164–4176. https://doi.org/10.1016/j.watres.2007.04.005
West, D., & Mangiameli, P. (2000). Identifying process conditions in an urban wastewater treatment plant. The International Journal of Operations and Production Management, 20(5), 573–590.
Xia, X., Meng, J., Zhao, S., Bodeis-Jones, S., Gaines, S. A., Ayers, S. L., & McDermott, P. F. (2011). Identification and antimicrobial resistance of extraintestinal pathogenic Escherichia coli from retail meats. Journal of Food Protection, 74(1), 38–44. https://doi.org/10.4315/0362-028X.JFP-10-251
Yang, M., & Zhang, X. (2013). Comparative developmental toxicity of new aromatic halogenated DBPs in a chlorinated saline sewage effluent to the marine polychaete Platynereis dumerilii. Environmental Science and Technology, 47(19), 10868–10876. https://doi.org/10.1021/es401841t
Young, H. K. (1993). Antimicrobial resistance spread in aquatic environments. Journal of Antimicrobial Chemotherapy, 31(5), 627–635. https://doi.org/10.1093/jac/31.5.627
Young, K. D., & Thackston, E. L. (1999). Housing density and bacterial loading in urban streams. Journal of Environmental Engineering, 125(12), 1177–1180. https://doi.org/10.1061/(ASCE)0733-9372(1999)125:12(1177)
Zerva, I., Remmas, N., Kagalou, I., Melidis, P., Ariantsi, M., Sylaios, G., & Ntougias, S. (2021). Effect of chlorination on microbiological quality of effluent of a full-scale wastewater treatment plant. Life, 11(1), 1–13. https://doi.org/10.3390/life11010068
Zhi, S., Banting, G., Li, Q., Edge, T. A., Topp, E., Sokurenko, M., & Scott, C. (2016). Evidence of Naturalized Stress-Tolerant Strains of Escherichia coli in Municipal Wastewater Treatment Plants. Applied and Environmental Microbiology, 82(18), 5505–5518. https://doi.org/10.1128/AEM.00143-16
Acknowledgements
The authors would like to thank the Municipality in NW-SA, for granting permission to get samples at their Wastewater Treatment Plant. The authors would like to appreciate financial support from the National Research Foundation (NRF), Grant number 112855.
Funding
Open access funding provided by University of South Africa.
Author information
Authors and Affiliations
Contributions
The following statements should be used “Conceptualization, S.M. and E.F.-K.; methodology, S.M., E.G. M.T., N.B. E.F.-K. and E.G.; validation, S.M., E.G. M.T., N.B. E.F.-K. and E.G.; formal analysis, S.M., E.G. M.T., N.B. E.F.-K. and E.G.; investigation, S.M.; resources, S.M. and E.F.-K.; writing—original draft preparation, S.M.; writing—review and editing, S.M., E.G. M.T. and E.F.-K.; supervision M.T., E.G. and E.F.-K.; project administration, S.M., M.T., E.F.-K. and E.G.; funding acquisition, E.G. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makuwa, S., Green, E., Tlou, M. et al. Molecular Classification and Antimicrobial Profiles of Chlorination-Resistant Escherichia Coli at Wastewater Treatment Plant in the North West Province of South Africa. Water Air Soil Pollut 234, 490 (2023). https://doi.org/10.1007/s11270-023-06484-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06484-5