Abstract
To mitigate lake eutrophication, phosphorus (P) availability can be managed by iron (Fe) amendments, which bind P in the water column and settle as Fe oxy-hydroxides. In the fluvial-lacustrine system Spree, Fe oxy-hydroxides enter lakes due to lignite mining in the Lusatian Area (NE Germany). We hypothesized that the amount of P that can be retained from the water column by sediments is positively correlated with their iron content. Column experiments were used to investigate uptake and release of P in the sediments under oxic and anoxic conditions in three downstream lakes (Lake Neuendorfer See, Lake Glower See, Lake Müggelsee) with decreasing mining influence and thus iron loads, and one nearby non-mining-affected lake (Lake Schwielochsee). In lakes interconnected by River Spree, the cumulative P uptake in sediments increased significantly with increasing sedimentary Fe concentrations under both oxic and anoxic conditions. Only the sediments of Lake Glower See had higher P uptake under anoxic than oxic conditions, most likely due to vivianite formation. The net P sedimentation was higher with higher Fe concentration and higher under oxic than anoxic conditions. However, the lakes are classified as eutrophic because although the sediments of the Spree lakes can store further P, this additional P uptake is of little relevance for the P budget in highly P-loaded lakes with short water residence times (10–100 d), as is typical for fluvial-lacustrine systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Eutrophication, a process of global concern, promotes plant and algal growth because of the increased availability of at least one of the limiting factors required for photosynthesis and growth, such as sunlight, CO2, and nutrients (e.g., phosphorus (P), nitrogen (N), and iron (Fe)) (Schindler, 2006; Sibrell et al., 2009).
The availability of P in lacustrine systems is controlled not only by external sources including riverine input, watershed runoff, sewage and industrial effluents as well as aeolian dust (Katsev, 2016; Markovic et al., 2019), but also by the retention of P within the sediments (Hupfer et al., 2016). The retention of P depends on sedimentation, sediment characteristics, redox conditions at the sediment–water interface (SWI), and geological conditions in the catchment area (Hupfer & Lewandowski, 2008; Moosmann et al., 2006). High Fe and aluminum (Al) concentrations or sedimentary contents can generally decrease the bioavailable P fraction by complexation or by rapid adsorption of P onto iron and aluminum oxy-hydroxides based on ligand exchange reactions, especially under oxic conditions (Kim et al., 2011; Sibrell et al., 2009; Spikjerman, 2008). Thus, previous studies have found clear positive correlations between sedimentary Fe and P contents (e.g., Jensen et al., 1992). The release of P from sediment (“internal loading of P”) is often related to the redox cycling of Fe including the reduction of Fe oxy-hydroxides and subsequent mobilization of Fe-coupled P under anoxic conditions (e.g., Mortimer, 1941; Katsev, 2016), although there are more mechanisms besides iron cycling that cause P release to the water column (Orihel et al., 2017).
Mining areas worldwide release large amounts of acid mine products, including Fe, so Fe from mining areas could generally alter the P dynamics in neighboring fluvial and lacustrine systems. The fluvial-lacustrine system Spree (NE Germany) is heavily impacted by Fe and sulfate (SO42−) loads from the Lusatian lignite mining area (Friedland et al., 2021; Gabriel et al., 2008). Heidenreich and Kleeberg (2003) and Gabriel et al. (2008) showed that settling iron oxy-hydroxides in low-flow areas of the River Spree (Reservoir Spremberg and Spreewald), are able to adsorb soluble reactive P (SRP), thus reducing the concentration of SRP in the water (Fig. 1). Adsorption of P also occurs when anaerobic groundwater with high Fe(II) and P concentrations becomes oxic at the SWI and Fe(III) precipitates as iron oxy-hydroxides (Zak et al., 2004). In the shallow eutrophic lakes of the River Spree, > 70% of the phosphorus in the sediments is adsorbed by iron minerals (Hupfer et al., 2002). Similarly, batch experiments showed extremely high P adsorption capacities compared to capacities found in natural lakes, with P adsorption dependent on Fe content in Lusatian mining lake (ML) sediments (Grüneberg & Kleeberg, 2013), and Reservoir Spremberg (Heidenreich & Kleeberg, 2003). These systems receive lignite mine products via ground and surface water. However, the iron load decreases with decreasing distance from the mining area and in the long-term after closure of the mines; thus, controlling the P binding capacity (Behrendt, 2002). It has already become apparent that these huge interventions in nature have several negative effects, such as changes in hydraulics and toxic effects on biota (Gabriel et al., 2008; Zak et al., 2021), and in contrast could also have positive effects on further eutrophication.
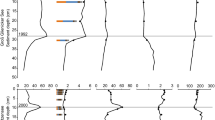
TFe and SRP concentrations along River Spree are shown with arithmetic means and standard deviations for the years 2015–2019. Calculations based on data provided by LfU (2019), LfU (2021b), LfULG (2019), LfULG (2022), SenUVK (2020), SenUVK (2021). A dataset of a sampling station with measurements n < 30 over the five years period have been corrected for outliers with Grubbs test in R (applies only to Fe data upstream of Reservoir Spremberg). Grey bars show areas of iron input
Previous studies worldwide have shown that iron amendments such as iron mine tailings and products can be useful for preventing P from entering surface waters (e.g., Sibrell et al., 2009; Wei et al., 2008), directly decreasing the SRP concentration in the receiving water (e.g., Simmons, 2010; Tate et al., 1995), or reducing internal P loading in lakes (e.g., Clayton et al., 2004; Tang & Nairn, 2021). However, none of the latter studies completely account for the internal structure of the sediments, the potential penetration depth of P under oxic and anoxic conditions, or penetration depth at relatively high sedimentary iron contents as is caused by mining. Therefore, this study used column experiments to study P uptake and release from the undisturbed sediments of four eutrophic to hypereutrophic lakes with different mining-caused Fe concentrations, under oxic and anoxic conditions. Sediments from shallow lakes along the River Spree or close by, with Fe concentrations ranging from relatively iron-poor (81 µmol cm −3 wet sediment in the upper 6 cm) to iron-rich (273 µmol cm −3, due to lignite mining in Lusatia). Column studies have been previously used to investigate P fluxes into and from sediments under oxic and anoxic conditions, e.g., in the eutrophic, non-mining affected Reservoir Bautzen along River Spree (Dadi et al., 2020). We hypothesized that (1) high sedimentary Fe content causes high cumulative P uptake and low P release, and (2) redox conditions alter P dynamics depending on hydrogen sulfide content and formation of vivianite, a stable Fe–P mineral under anaerobic conditions. Phosphorus availability and productivity, particularly in shallow lakes, depend on short-term (seasonal) storage and release (Hupfer et al., 2019b). As periods of anoxic conditions at the water surface of shallow lakes increase with climate change (Golosov et al., 2012), we hypothesized that (3) previously adsorbed P is released in large amounts under anoxic conditions.
Schulz and Herzog (2004) were critical of studies on sorption processes in running waters that lacked quantification of P sorption in relation to the P mass balances of entire river sections. Therefore, based on the results of our column experiments and P loads within the lakes, we discuss the overarching question of whether the mining-caused sedimentary Fe content can cause a lower trophic state of the lakes. We give special consideration to the reactions of iron under different redox conditions and the P-binding forms within the sediment.
2 Material and Methods
2.1 Study Area and Sediment Sampling
The River Spree in northeastern Germany is a 397 km long lowland river flowing from the Lusatian Mountains near the Czech-German border to the River Havel in Berlin. It is intersected by reservoirs (e.g., Bautzen and Spremberg), several polymictic and shallow lakes (e.g., Neuendorfer See, Glower See, and Müggelsee), and the inland delta Spreewald. Further information on the morphology, hydrodynamics, and other characteristics can be found in Köhler (1994) and Gabriel et al. (2008).
The water quality of the River Spree is strongly affected by lignite mining in the Lusatian area. Iron and other metals, SO42−, and acids are transported towards the Spree and smaller tributaries by rainwater and percolating water, the re-rise of groundwater, and the flooding of open-pit mines after closure of the mines. These mining products originate from the weathering of iron sulfide minerals associated with lignite (Friese et al., 1998). There are two input areas for iron and sulfate, which are located south of the Reservoir Spremberg, (“southern area”) and south of the Spreewald (“northern area”) (Friedland et al., 2021). Moreover, iron and manganese concentrations are naturally elevated in groundwater in northern Germany (Busskamp, 2013). Other anthropogenic influences on the River Spree include urban and agricultural uses (Friedland et al., 2021; Nützmann et al., 2011). Phosphorus sources and transport processes in the approximately 10,000 km2 catchment area of the River Spree (Köhler, 1994) include municipal waste water treatment plants (WWTP) and swamp water (47%), urban areas (18%), groundwater (15%), and erosion (12%), among others (Behrendt, 2002).
The SRP concentration strongly decreased when iron enters the Spree before the Reservoir Spremberg and in the Spreewald (Fig. 1). After Spreewald, the SRP concentrations increased further downstream with decreasing Fe concentrations, particularly under the influence of the urban area of Berlin. In this study, sediments from Lake Neuendorfer See, Lake Glower See, Lake Gr. Schwielochsee (in rural area), and Lake Müggelsee (urban area of Berlin) were used. The lakes are listed in order according to their sedimentary iron concentrations, from high to low (Tab. 1). The locations of the lakes within the river are shown in Figs. 1 and 2. A decreasing trend of iron contents in the selected lakes was found previously, as reported in the studies of Kleeberg et al. (2013) and Friedland et al. (2021).
a) Location of the investigated lake sediments along River Spree. b) Lake Neuendorfer See is located closest to the Lusatian lignite mining area. Further downstream, River Spree flows through c) Lake Glower See and d) Lake Müggelsee at the city border of urban Berlin. Lake Schwielochsee (c) was used as a control lake with much lower sedimentary iron contents
Lake Neuendorfer See and Lake Glower See are the first two lakes located about 22 and 49 km downstream of the Spreewald region. The P dynamics in the River Spree and the Spreewald region (source or sink) influence the P input into the downstream lakes (Gabriel et al., 2008). Lake Glower See is connected to the northern end of Lake Gr. Schwielochsee and discharges into Lake Leisnitzer See. However, most of the Spree water (approximately 90%) flows only through Lake Glower See and not through Lake Schwielochsee owing to the closure of a second tributary to Lake Schwielochsee in 1910 (Driescher, 2002; Köhler et al., 2002). Therefore, Lake Schwielochsee served as control site with Fe poor sediments (Table 1). Lake Müggelsee is located approximately 90 km downstream of Lake Glower See. All lakes are shallow and polymictic. Henceforth, the three lakes Lake Neuendorfer See, Lake Glower See, and Lake Müggelsee will be referred to as the “iron-enriched” lakes.
Twelve undisturbed sediment cores were taken from an area of 5 m2 within each lake using a modified UWITEC corer (Uwitec, Mondsee, Austria) in 40 cm transparent PVC sediment cores (inner diameter 59 mm) on 8th and 9th of April 2021 (Fig. 2b–d). Seven of the twelve cores were previously prepared with holes at 1 cm intervals, allowing for pore water analysis with rhizon samplers (Shotbolt, 2010) later during the experiment. During sampling, we also used a sediment grab sampler and sieve to check for the presence of chironomid larvae (Diptera, Chironomidae).
2.2 Column Experiment: P Uptake and Release Under Oxic and Anoxic Conditions
2.2.1 Experimental Setup
We conducted a column experiment investigating the reactivity of sedimentary Fe towards P fixation under constant temperature and redox conditions, neglecting other factors such as the continuous import of particulate organic matter and advective transport through resuspension along the SWI.
In the laboratory, the SWI was set at the same height in all cores: 15 ± 0.5 cm sediment and 25 cm overlying water. The overlying water in all columns was then removed, and the columns were refilled by bank-filtrated raw water from Lake Müggelsee to ensure equal starting conditions in all columns. The raw water contained 0.19 mg L−1 SRP, < 0.01 mg L−1 dissolved Fe, 206 mg L−1 SO42−, 0.01 mg L−1 NO3−-N and 0.66 mg L−1 NH4+-N. All columns were then closed with a lid, which contained a small hole for a gas tube. The columns were then placed in a climate chamber adjusted to 12 °C, which is only slightly higher than the mean River Spree temperatures reported by Wanner et al. (2002); thus, the chamber was about 5 °C warmer than the water during sediment sampling.
The sediments (12 columns per lake) were pre-incubated by continuous flushing with a N2-CO2-gas mixture for 28 days, which resulted in a decrease in oxygen concentration in the overlying water < 2% air saturation. Surface sediments might have been disturbed by transportation, water change, or sediment height correction and were then able to settle down. At the end of the pre-incubation phase, two cores from each lake were used for P fractionation, pore water analysis (Sect. 2.2.3), water column analysis (Sect. 2.2.2), and bulk parameter sediment analysis (Sect. 2.2.2), to represent the starting conditions. Online Resource A contains a full scheme showing the usage of each of the twelve cores during the full experiment.
Phase A of the main experiment was to determine the maximum cumulative P uptake (n = 5). For this, 3–5 mL of overlying water was sampled with a glass tube twice a week and the columns were then refilled using filtered bank filtrate (pre-rinsed cellulose acetate (CA) filters, 0.45 µm, Sartorius, Germany) and an SRP stock solution (from KH2PO4), until the SRP in the water was readjusted to 4 mg L−1. At this concentration, the conditions in the pore water were approximately mimicked (Hupfer et al., 2019a).
Other parameters, namely Fe, other metals, NO3−-N, NH4+-N, and SO42−, were measured less frequently (Sect. 2.2.2). Half of the cores of each lake were treated under oxic conditions with continuous aeration, and the other half were continuously bubbled with an N2-CO2-gas mixture, or only partial bubbling with N2. Both oxic and anoxic treatments were investigated for all four lakes, with five replicates each. After phase A, two cores from each lake and treatment were used for P – fractionation and pore water analysis. The termination criteria are described in Sect. 2.2.4.
Phase B of the experiment was to determine P release. Half of the cores of each lake were again treated under oxic conditions with continuous aeration, and the other half with continuous bubbling with an N2-CO2-gas mixture or only partial bubbling with N2. During this release phase the overlying water in each core was exchanged with filtered bank-filtrated water from Lake Müggelsee (three replicates for each treatment). More precisely, every 2–4 weeks, the SRP concentration in the water was analyzed photometrically and restocked to approximately 0.3 mg L−1 using bank-filtered water without P addition. The other parameters (Fe, other metals, NO3−-N, NH4+-N, and SO42−) were remeasured before rediluting the SRP concentration. After phase B, the final P fractionation and pore water analysis were performed for each treatment.
2.2.2 Water Column Analysis
The overlying water was carefully sampled with a glass tube over the full water column depth and immediately filtered through a 0.45 µm syringe filter (30/0.45 CA, Whatman Puradisc). The SRP concentration was measured using a photometer (SPEKOL, Analytik Jena AG, Jena, Germany) using the molybdenum blue method (DIN EN ISO 6878). The metals were analyzed using inductively coupled plasma-optical spectrometry (ICP-OES, iCAP7000 series, Thermo Fisher Scientific, Waltham, MA, USA). NH4+-N and NO3−-N were determined using a continuous flow analyzer (CFA Skalar SAN++ AA3, Seal Analytical, Norderstedt, Schleswig–Holstein, Germany). The detection limits for ICP-OES measurements, NH4+-N and NO3−-N were 0.01 mg L−1. Sulfate was measured using ion chromatography (Compact IC Flex 930, Metrohm, Herisau, Switzerland) after filtering the water through a 0.2 µm filter (Minisart NML, CA, Sartorius). Additionally, physicochemical parameters such as pH, oxygen concentration, temperature, and electrical conductivity were measured every two weeks.
2.2.3 Sediment and Pore Water Analysis
Bulk sediments were sliced into horizons (0–1 cm, 1–3 cm, 3–6 cm, 6–10, 10–15 cm). The horizons were analyzed for their dry weight (dw, 105 °C until constant weight after DIN EN 12880) and loss on ignition (450 °C, 3 h).
Sequential P extraction according to Psenner et al. (1984) modified by Hupfer et al. (1995) was conducted in the three upper layers to differentiate between the different P-binding forms in the sediments (Table 2). More precisely, these are loosely adsorbed P/pore water P (NH4Cl-P), redox-sensitive bound P (BD-P), P bound to metal oxides (NaOH-SRP), P released from organic materials (NaOH-NRP), P in carbonates (HCl-P), and residual P (Rest-P). In the BD-P and HCl-P fractions, the dissolved iron was analyzed using atomic absorbance spectroscopy (AAS PinAAcle 900 T, PerkinElmer, Waltham, MA, USA). One core per treatment was examined at the beginning and the end of each experimental phase and used for the P sequential extraction, so the P sequential analysis could only be used to estimate the P penetration depth and the P binding forms. The yield of the sum of the P fraction compared with the total P content measured via digestion was at least 70%.
Aliquots of fresh material were freeze-dried and homogenized with an agate mortar for trace metals, sulfur (S), total organic carbon (TOC), total nitrogen (TN), and TP. TOC, N, and S were analyzed with an elemental analyzer (Vario EL, Elementar, Langenselbold, Germany). Metals were determined after wet digestion with reverse aqua regia (37% HCl, 65% HNO3, 1:3 v/v) in a high-pressure microwave oven (µPREP-A; MLS GmbH, Frankfurt am Main, Germany). The concentrations were then measured using ICP-OES. Total phosphorus (TP) was measured photometrically with the ammonium molybdate method (DIN EN ISO 6878) after digestion with 2 mL of H2O2 (5%) and 2 mL of H2SO4 (5 M), similar to the procedure of Kleeberg et al. (2010). Element contents were converted from mg g−1 dw to element concentrations in µmol cm−3 taking the different dry weights (9–29%) and the loss on ignition (8–29%) into account and assuming a density of the organic material of 1.4 g cm−3 (porg) and of inorganic compounds of 2.65 g cm−3 (pinorg). The conversion was done based on Eqs. 1–4:
Calculation of density of solid matter (psol, g cm−3):
Calculation of density of wet sediment (pwet, g cm−3) with density of water (pw):
Calculation of mass of solids (msol) per volume wet sediment (Vwet, g cm−3):
Conversion of mass content (g g−1) to molar mass content (mol g−1):
This was because the P uptake and release are dependent on the mass of solid per volume of a specific layer and not on the mass of solid per mass of dry weight. Additionally, it allows for a better comparison of the Fe and P content within a specific layer.
Qualitative mineral analysis of the crystalline mineral phases was carried out using X-ray diffraction (XRD). Therefore, a D2Phaser (Bruker, Billerica, MA, USA) with a cobalt X-ray tube was used and was equipped with a 1 mm fixed divergence slit, a 2,5° primary and 4° secondary soller collimator, an air-scatter device 3 mm above the surface sample, and an Fe k-beta filter (2.5). Measurements were carried out from 10–90° 2Theta with a measurement time of 6 h. The subsequent phase analysis was carried out with the DIFFRAC.EVA V5.2 (Bruker, Billerica, MA, USA) using the Crystallography Open Database (Grazulis et al., 2012).
Pore water was extracted at 1 cm depth intervals with rhizon samplers (5 cm porous part, CSS, pore size 0.15 µm, rhizosphere, Netherlands) between 2 cm above the SWI up to 11 cm depth. It was then analyzed for SRP, metals, NO3−-N, NH4+-N, SO42−, as described above. Sulfide in H2S was analyzed after fixing immediately with 1% zinc acetate photometrically, according to the method of Cline (1969).
2.2.4 Calculation of Cumulative P Uptake and Release, and Statistical Analyses
The P uptake and release rates in each column were calculated using a mass-balance approach, which accounted for the change in SRP concentration between the two sampling dates in the water column (Eq. 5):
C represents the SRP concentration at time t1 and t0 (mg L−1), V the volume of the water column (L), A the area of surface sediment (m−2) and d the time between two sampling dates (days).
The cumulative uptake (mg P m−2) was reached when the uptake rate approached zero, and the cumulative uptake stagnated at a high level. Correspondingly, maximum release was reached when no further P was released. Net sedimentation was calculated as the cumulative P uptake minus the cumulative P release (g m−2).
Statistical analyses were performed using the non-commercial program R (R Core Team (2017), version 4.0.5). The dependency of P contents on other elements in the sediments were tested using linear regression. When the different lakes were compared according to their Fe concentrations in the column experiment, the Kruskal–Wallis-Test with post hoc tests were used with the Benjamini–Hochberg method for p adjustment. We used the Mann–Whitney U test to compare the oxic and anoxic treatments of one lake. Differences were considered significant when the p-value was < 0.05.
2.3 Calculation of P Loads in Lake Neuendorfer See
P and Fe loads and net sedimentation were calculated for Lake Neuendorfer See for the years 2015–2020 for validation and better comparison of the experimental results with natural conditions. For the other three lakes, equivalent values, although for different periods, were obtained from the literature. Loads (g P m−2 yr−1) were calculated as \(L= \frac{Q*c}{A}\) (Eq. 6), with Q representing the discharge (L s−1), c the TP concentration (µg L−1), and A the lake area (m2) (Table 1). Loads were then corrected for their discharge \({\mathrm L}_{\mathrm{corr}}=\mathrm L\ast\frac{Q\;\mathrm{average}\;\mathrm{of}\;1\;\mathrm{year}}{Q\;\mathrm{average}\;\mathrm{of}\;\mathrm{all}\;\mathrm{measurement}\;\mathrm{days}}\) (Eq. 7) according to LAWA (2003) to account for daily measurements of discharge but non-daily measurements of concentrations. Water fluxes via evaporation, precipitation, groundwater, and bank filtration were not considered. The theoretical water residence time tR was calculated as \({t}_{R} = \left(\frac{V}{{Q}_{out}}\right)\) with V (volume of the lake) after Vollenweider and Kerekes (1982) (Eq. 8). To calculate the net sedimentation (g P m−2 yr−1), we used the relationships given by Hupfer and Scharf (2014) and a stratification factor ß of 1.
Discharge data were provided by LfU (2021a) and measured approximately 3 km upstream of the lake (299.6*106 m3 yr−1; period 2015–2020). It was assumed that Qout is equal to Qin because the water residence time was short (< 10 days). The TP and TFe concentrations (LfU, 2019; 24–26 measurements per year) were measured at the same location as the inflow discharge (LfU, 2021b). TFe and TP concentrations were directly measured at the outflow of the lake by LfU (2019) and LfU (2021b) (12 measurements per year).
3 Results
3.1 Lake Sediment Composition
The differentiation of lake sediments was based on the composition of the upper three layers (6 cm, weighted mean, Table 3). For detailed data on the layers, the reader is referred to Online Resource B.
The Fe concentration in the sediments was highest in Lake Neuendorfer See (273 µmol cm−3) and decreased in the sediments with further distance from the lignite mining area (Lake Glower See (201 µmol cm−3 and Lake Müggelsee (135 µmol cm−3)). The lowest Fe concentration was present in Lake Schwielochsee (81 µmol cm−3). Also noticeable were the high Fe pore water concentrations in Lake Neuendorfer See that were partly more than 40 mg L−1 at a depth of 5 cm compared to max 10 mg L−1 in the other three lakes (Online Resource C). The molar Fe:P pore water ratio was constantly < 1.2 in the upper 6 cm only in Lake Müggelsee, whereas it reached up to 56 in sediments of Lake Neuendorfer See.
The reductively soluble Fe concentration decreased downstream from 29% in Lake Neuendorfer See to a level of 10–12% in the other three lakes. In contrast, the HCl-Fe concentration was at least 50%, apart from Lake Schwielochsee, where the HCl-Fe concentration was approximately 32%.
The P concentration before the experiment was the highest in Lake Neuendorfer See (26 µmol cm−3), followed by Lake Glower See (20 µmol cm−3), Lake Müggelsee (12 µmol cm−3), and Lake Schwielochsee (10 µmol cm−3). An equal molar Fe:P ratio of 10–11 was found in all three mining impacted lakes, and a lower ratio of approximately 8.4 was found in Lake Schwielochsee. There was a highly significant linear correlation between TFe and TP (F(1,10) = 152.7, R2 = 0.94, TP = 0.0847 TFe + 2.457, p < 0.001) across all sediments (Fig. 3a).
At the same time, there was also a clear positive linear relationship between the BD-Fe concentration and the BD-P concentration (F(1,10) = 27.55, R2 = 0.74, BD-P = 0.127 BD-Fe + 3.79, p < 0.001). In all lakes, the BD-P fraction was highest, however, the relative amount of P in the BD-P fraction decreased in the following order: Lake Neuendorfer See (69%), Lake Glower See and Müggelsee (50–51%) and Lake Schwielochsee (38%). In Lake Neuendorfer See and Lake Glower See, the second biggest fraction was the NaOH-SRP, which includes all P that was bound to non-reducible metal oxides. While in Lake Neuendorfer See this fraction was approximately 17%, in Lake Glower See the amount of NaOH-SRP increased to approximately 35% at the cost of BD-P. In Lake Müggelsee, each of the NaOH-SRP, NaOH-NRP, and HCl fractions were between 14 and 17%. In Lake Schwielochsee, the second biggest P fraction was NaOH-NRP (28%).
Sedimentary S concentration was highest in Lake Schwielochsee (98 µmol cm−3) and lowest in Lake Müggelsee (26 µmol cm−3). Sediments of Lake Glower See contained 45 µmol cm−3 S, and sediments of Lake Neuendorfer See contained 40 µmol cm−3 S. This led to molar S:Fe ratios in Lake Schwielochsee of approximately 1.2 and in the other three lakes the molar S:Fe ratio was < 0.3.
In addition to Fe, other metals were also present in the different lake sediments. In Lake Neuendorfer See, among the metals, the order of most abundant metals in the upper layer was Fe > > Al > Ca > Mg > Mn, whereas in Lake Glower See the order was Fe > > Ca > Al > Mg > Mn. In Lake Müggelsee and Lake Schwielochsee, the order was Ca > > Fe > > Al > Mg > Mn. Taking all sediments into account, in addition to the positive correlation between Fe and TP, there was a clear positive linear correlation between Mn and TP (F(1,10) = 82.73, R2 = 0.89, TP = 2.0786 Mn + 4.126, p < 0.001) and Al and TP (F(1,10) = 19.24, R2 = 0.66, TP = 0.332 Al—2.515, p = 0.001), (Fig. 3b,c). Additionally, there was a highly significant positive correlation between TFe and Mn concentrations (F(1,10) = 42.3, R2 = 0.81, Mn = 0.035Fe + 0.0218, p < 0.001). On the other hand, sediments of Lake Neuendorfer See and Lake Glower See had higher TP together with lower Ca concentrations, and sediments of Lake Müggelsee and Lake Schwielochsee had higher Ca with lower TP concentrations (Fig. 3d).
All sediments contained quartz, calcite, minerals of the feldspar group and pyrite, among others, which were not further identified. No vivianite was detectable by XRD in the starting sediments of Lake Schwielochsee and Lake Neuendorfer See. In contrast, vivianite was found by XRD at a depth of 3–6 cm in Lake Müggelsee and in Lake Glower See (1–6 cm depth).
3.2 Column Experiment P Uptake and Release
Aeration led to oxygen levels > 90% in the water column and to an increasing brownish layer at the sediment surface, depending on the sediment type (Lake Schwielochsee: 0.8 ± 0.2 cm, Lake Müggelsee 1.2 ± 0.1 cm, Lake Glower See 0.8 ± 0.1 cm, Lake Neuendorfer See 1.1 ± 0.05 cm). Bubbling with the CO2-N2-mixture in the anoxic treatments led to oxygen air saturation levels < 5% oxygen air saturation and a < 1 mm orange flaky layer at the SWI of samples from Lake Neuendorfer See, Lake Glower See, and Lake Schwielochsee, within a few days, but not in those from Lake Müggelsee. Additionally, in Lake Schwielochsee, chironomid 4th instar larvae (Diptera, Chironomidae) were present and active in the anoxic starting phase and later on permanently under oxic conditions and in the beginning of the anoxic uptake phase. Activity was observed in the form of burrow tubes up to a depth of 10 cm with an oxidized surface area. The burrow walls visibly decreased over time under anoxic conditions. pH in all sediments was mostly within the range of 7.9 to 8.5; pH in the anoxic treatment was always slightly lower than in the oxic treatment.
3.2.1 P Uptake Rates and Cumulative P Uptake
The cumulative uptake of P by sediments of Lake Neuendorfer See was higher under oxic than under anoxic conditions (26.57 ± 5.64 g m−2 compared to 9.51 ± 0.75 g m−2, respectively), and the difference was significant (W = 0, n = 5, p = 0.008) (Fig. 4a). The Fe:P ratios in the upper 6 cm decreased during the incubation from the initial 10.6 to 6.6 under oxic and 9.5 under anoxic conditions. The P uptake rates under oxic conditions remained high (> 100 mg m−2 d−1) during the entire experiment, whereas the rates under the anoxic treatment decreased linearly with time starting from 128 mg m−2 d−1. In the last week of the P uptake phase of the anoxic treatment, the pH increased from 8.2 to 8.9 due to N2 usage alone and contemporaneous with the increase in P uptake rate by a factor > 5. Nevertheless, we stopped the uptake phase at this point (Online Resource D).
Results from column experiments. a) Cumulative P uptake (n = 5) and b) cumulative P release (n = 3) in the four different lakes differentiated for oxic and anoxic treatments. c) Net sedimentation within columns (mean cumulative uptake minus mean cumulative release). Please note that the cumulative release is smaller by a factor of 10 than the cumulative P uptake
We determined a cumulative P uptake in Lake Glower See of 2.71 ± 0.29 g m−2 under oxic and 3.20 ± 0.17 g m−2 under anoxic conditions (Fig. 4a). The difference was significant (W = 23, n = 5, p = 0.03, two-tailed). The Fe:P ratios in the upper 6 cm decreased during the incubation from an initial 10.1 to 8.2 under oxic and to 7.8 under anoxic conditions. Phosphorus uptake rates are shown in Online Resource D.
The sediments of Lake Müggelsee showed the lowest cumulative P uptake (oxic: 1.23 ± 0.10 g m−2; anoxic: 1.04 ± 0.07 g m−2; Fig. 4a). Thus, P uptake was significantly higher in the oxic treatment than in the anoxic treatment (W = 1, n = 5, p = 0.016, two-tailed). This resulted in new Fe:P molar ratios for the upper 6 cm: 9.4 under oxic and 10.5 under anoxic conditions. As also observed in Lake Glower See and Lake Schwielochsee, the P uptake rates decreased linearly in both treatments (Online Resource D).
The cumulative P uptake in Lake Schwielochsee was 5.79 ± 1.67 g m−2 under oxic and 1.98 ± 0.48 g m−2 under anoxic conditions (Fig. 4a). The difference between treatments was statistically significant (W = 0, n1 = 5, n2 = 4, p = 0.016, two-tailed). As a result, molar Fe:P ratios in the upper 6 cm during the uptake phase reached 6.6 under oxic and 7.8 under anoxic conditions. In both treatments, the P uptake rates decreased linearly; however, in the oxic treatments, we ended the uptake phase after 3 weeks of a constant uptake rate of 40 mg P m−2 d−1 (Online Resource D). Only within the oxic treatments did a minimum of one and a maximum of four chironomid larvae emerge per column during phase A. Subsequently, the visible amount of new burrow walls decreased, and the existing burrow walls started to refill with sediment.
In summary, the cumulative P uptake in the sediments was strongly correlated with sedimentary iron concentration (Fig. 4a). Kruskal–Wallis tests showed highly significant differences between the iron-enriched lakes for both oxic and anoxic treatments (oxic: X2 = 12.5, df = 2, p < 0.001; anoxic: X2 = 12.5, df = 2, p < 0.001, one-tailed). Additionally, post-hoc tests between the groups confirmed significant differences between sediments of the control and the iron-enriched lakes and in oxic and anoxic treatments (p < 0.05 in each combination). We excluded Lake Schwielochsee from this calculation because we observed the presence and activity of chironomid larvae, which alters P uptake in sediments (Hupfer et al., 2019a).
Water chemistry data collected during phase A can be found in Online Resource E.
3.2.2 P Release Rates and Cumulative P Release
In the second phase of the experiment, the amount of P, which was not permanently stored in the sediments and released from the sediments, was investigated when the P concentration in the overlying water column was low (0.3 mg L−1). The cumulative release in the iron-enriched lakes was not significantly higher with decreasing iron concentration (oxic: X2 = 5.11, df = 2, p = 0.08, two-tailed; anoxic: X2 = 5.95, df = 2, p = 0.05, two-tailed, Fig. 4b).
In the sediment samples from Lake Neuendorfer See, no P was released in phase B under oxic conditions (instead even more P was taken up) and 0.41 ± 0.14 g m−2 P was released under anoxic conditions. The P release from Lake Glower See was lower under oxic than under anoxic conditions (0.06 ± 0.08 g m-2 compared to 1.43 ± 0.44 g m−2; W = 9, n = 3, p = 0.1). The amount of P released from the sediment samples from Lake Müggelsee was significantly lower under oxic conditions (0.17 ± 0.04 g m−2) than under anoxic conditions (1.26 ± 0.37 g m−2; W = 1, n = 3, p = 0.02). In the sediment samples from Lake Schwielochsee, the cumulative P release under anoxic conditions (1.81 ± 0.72 g m−2) was higher than under oxic conditions (0.12 ± 0.04 g m−2; W = 6, n = 3, p = 0.2). In summary, the cumulative release of P was higher under anoxic conditions than under anoxic conditions for all the lakes, independent of the iron concentration. The P release rates are shown in Online Resource F.
A comparison of the cumulative P uptake in phase A and the release in phase B revealed that a higher iron content in the lake sediments increased the relative amount of P that was stored permanently (higher net sedimentation) (Fig. 4c).
3.2.3 P Binding Forms in the Sediments
BD-P and NaOH-SRP are the most important fractions in the context of this study, as these fractions are directly related to the P that is bound to reductively soluble minerals such as iron hydroxides, and the P bound to metal oxides. Figure 5 shows the BD-P and NaOH-SRP concentrations at the start and after the uptake and release phases under oxic and anoxic conditions, as well as the BD-P:NaOH-SRP ratio.
Comparison of P in BD-P, NaOH-SRP and BD-P:NaOH-SRP ratio at the start, the P uptake and release phase. Deeper layers show a tendency towards higher NaOH-SRP concentrations. Checkmarks mark the qualitative found of vivianite with XRD in a layer. We assume that an increase in NaOH-SRP content is a strong indicator also for the increase in vivianite content as 84% of synthetic vivianite was extractable with NaOH-SRP in Rothe et al. (2015)
Based on these results, three general observations can be made:
-
(i)
An increasing iron concentration in the sediments correlates with an increase in P in these two fractions (indicated by the pyramidal shape of the graph). The sum of BD-P and NaOH-SRP as a percentage of the total sum of P within the fractions was between 33–80% in Lake Schwielochsee, 59–75% in Lake Müggelsee, 79–89% in Lake Glower See and 82–93% in Lake Neuendorfer See, considering both phases and both redox states.
-
(ii)
The upper layers tended to have higher BD-P concentrations than the lower layers, and the lower layers tended to have higher NaOH-SRP concentrations than the upper layers (visible in the BD-P:NaOH-SRP ratio).
-
(iii)
There were tendencies for NaOH-SRP to increase in anoxic layers compared to oxic layers, and for higher BD-P concentrations in oxic sediments compared to anoxic layers (indicated by the parallelogram shape of the oxic and anoxic layers together).
In Lake Schwielochsee, only the uppermost layer showed an increase in BD-P under oxic and anoxic conditions, but no change was observed in NaOH-SRP. No vivianite was detected under oxic nor anoxic conditions by XRD analysis, neither after phase A nor after phase B.
In the sediments of the Müggelsee, the decrease in the BD-P concentration and the steady increase in the NaOH-SRP concentration in the 1–3 cm layer were particularly noticeable (indicated by a comparison of values at the start, phase A, and phase B under anoxic conditions).
In the sediments of Lake Glower See, a strong increase in the NaOH-SRP concentration in the anoxic treatment in the top centimeter in phase A could be observed together with an increase in the height of the vivianite reflections (Online Resource G), and in depths > 1 cm in phase B. In phase B, an increase in the NaOH-SRP concentration in the oxic treatment could also be observed at a depth of 1–6 cm, whereas the BD concentration decreased, similar to observations of the Lake Müggelsee sediments.
Most prominent in the sediments of Lake Neuendorfer See was the strong increase in the NaOH-SRP concentration from the start to phase B in the anoxic treatments and at all depths. Vivianite formation could be clearly observed using XRD. An example is provided in Online Resource G. No vivianite was encountered under oxic conditions.
The HCl extractable P fraction (mainly Ca-bound P) was negligible in sediments from Lake Glower See and Lake Neuendorfer See (1–5%), whereas this proportion increased to 9–18% in the sediments of the Müggelsee and even reached 26% in Lake Schwielochsee.
3.2.4 Pore Water Measurements
Figure 6 shows pore water data (Fe, Mn, SRP and H2S-S) of the different lakes down to 6 cm depth. The trends presented in the following refer to median values of a mentioned group if not otherwise declared. Median Fe and Mn concentrations were higher at the start of the experiment and decreased in the iron-enriched lakes during the experiment (Fig. 6a,b). Fe pore water concentrations of the oxic and anoxic treatments in Lake Neuendorfer See and Glower See (16.8 to 2.46 mg L−1) were higher than in Lake Müggelsee and Lake Schwielochsee (2.18 to 1.3 mg L−1, Fig. 6a). In all lakes, oxic overlying water led to lower Mn pore water concentrations in the upper 6 cm depth. SRP concentrations increased in all sediments due to SRP addition over time. The concentrations were highest in Lake Schwielochsee under oxic conditions (4746 µg L−1) and smallest in Lake Neuendorfer See under oxic conditions (743 µg L−1, Fig. 6c). Further, SRP in the pore water of oxic treatments were generally lower compared to SRP of the anoxic treatments in the iron-enriched lakes. H2S-S concentrations in the pore water were highest in the sediments of Lake Schwielochsee (measurement data up to 1.5 mg L−1) and lower in the iron-enriched lakes (often lower than the smallest standard, < 0.125 mg L−1, Fig. 6d).
Pore water concentrations of Fe, SRP, Mn, and H2S-S until 6 cm depth in the different sediments at the start, under oxic and anoxic conditions. The boxplots for oxic and anoxic data points include both, data from measurements after the uptake and the release phase. Aluminum measurements are not presented as most of the measurements are below the detection limit as dilution of the small pore water amounts were necessary before ICP-OES analyses. Depth distributed pore water data in the starting sediments can be found in Online Resource C
3.3 P Loads in the Fluvial-Lacustrine System
Table 4 summarizes the calculated P loads for Lake Neuendorfer See (2015–2020) and P loads and water residence times for the investigated lakes as reported in the literature. If available, net sedimentation rates were also included. The largest external surface loads were given in Lake Glower See and the lowest in Lake Müggelsee. The shorter water residence times of Lake Neuendorfer See and Lake Glower See compared to those of Lake Müggelsee and Lake Schwielochsee are striking.
4 Discussion
4.1 Phosphorus Uptake Enhanced by Sedimentary Iron: Field-Scale Evidence
The sediments from the Spree lakes used in this study were relatively enriched with Fe compared to the sediments of Lake Schwielochsee. Mining-impacted sediments can generally be considered Fe-rich, as they contain more than 5 wt% iron (Rickard, 2012, c.f. Online Resource B).
Under in-lake conditions, higher Fe concentrations also led to higher P concentrations in sediments (Table 3, Fig. 3), with molar Fe:P ratios of approximately 10–11 in the sediments of the iron-enriched lakes. However, the three lakes had only slightly higher molar Fe:P ratios than other neutral lakes not affected by mining (molar Fe:P ratios approximately 8) and not nearly as high as in neutral ML without unusual P loading (17–148) (Grüneberg & Kleeberg, 2013). According to Jensen et al. (1992) a sedimentary molar Fe:P ratio above 8.3 is probably most effective to control the P release from surface sediments. Chen et al. (2016) reported the necessary ratios to be even > 9.9. Sediments of Lake Schwielochsee were the only ones in this study that hardly reached these threshold values in the surface sediments at the start of the experiment (molar Fe:P 8.4).
In addition to Fe, Al and Mn were positively correlated with P (Fig. 3a–c). Aluminum has similarly high absorption capacities for P as Fe (Hansen et al., 2003); however, given the fact that the sedimentary Fe concentration was > > Al > > Mn, the Fe concentration must be the dominant factor influencing P retention. The same conclusion was drawn for other lacustrine sediments by Bortleson and Lee (1974). Like Fe, Al and Mn are introduced anthropogenically into the lakes via the Spree as a consequence of mining next to the natural background and they precipitate as oxy-hydroxides alongside other already existing minerals such as clay minerals (Friedland et al., 2021). In contrast, calcium decreased with increasing P content. Iron dominates the P-binding behavior as adsorption of P takes place onto iron oxy-hydroxides, even when the sediments have high carbonate contents (Golterman, 1988). However, in addition to sorption processes, the simultaneous precipitation of calcium and phosphate as negative feedback to lake eutrophication (Hamilton et al., 2009; Koschel et al., 1983) can occur due to the consumption of CO2 by phytoplankton, which increases the pH, resulting in the co-precipitation of P by carbonates. This process was at least suspected in Lake Müggelsee (Kozerski & Kleeberg, 1998; Schmidt et al., 2019).
4.2 Phosphorus Uptake and Release in Studied Lake Sediments Related to the Sedimentary Iron Concentration: Column Study
4.2.1 General Behavior
The column study showed that P uptake increased with sediment Fe concentration independent of redox conditions (Fig. 4). Therefore, all sediments could retain more P. This demonstrates that sediment surface sediments do not necessarily have to be oxic to adsorb or retain P also under anoxic conditions, sorption sites for P are present or P can be retained via other processes (e.g., via mineral formation).
Under oxic conditions, all sediments acted as P net sinks because the P uptake in phase A was higher than the P release in phase B. This observation can be explained by the presence of iron or other metal oxy-hydroxides in the uppermost surface layer, and was shown by the increase in total BD-P (Fig. 5) in nearly all surface sediments. The Fe oxy-hydroxides remained stable under oxic conditions. Fe2+ diffusing from below the oxic layer towards the SWI can generally be re-oxidized in the oxic zone of the sediments. This phenomenon is known as the “iron wheel “ (Grüneberg & Kleeberg, 2013). Then, Fe is rarely released back to the overlying water (measured Fe concentrations were near or below the detection limit in the overlying water, see Online Resource E).
On the other hand, under anoxic conditions, P being released back to the water column when no further P was delivered showed that, depending on the Fe content, the sediments were clearly net P sources at lower Fe contents (Lake Müggelsee) and P sinks at higher Fe contents (Lake Glower See/Lake Neuendorfer See). Lake Schwielochsee had a very low positive net sedimentation. This illustrates that firstly, under anoxic conditions, previously retained P is not always completely released back to the water column, and secondly, with higher iron content, the sediments are less susceptible to redox-driven dynamics. This can be attributed to several factors. First, the thermodynamic stability of the Fe(OH)3-P structure is higher than that of pure Fe(OH)3; therefore, a transformation of ferric Fe to FeSx is necessary before P releases from Fe(OH)3-P (Golterman, 1995). Iron oxy-hydroxides can also remain in deeper sediments when Mn re-oxidizes Fe2+ in pore water to ferrihydrite or other ferric iron minerals (Herzsprung et al., 2010). This could also be the case in the sediments of this study, as there was a good correlation between Fe and Mn, and sediments of Lake Glower See and Lake Neuendorfer See had much higher sedimentary Fe and Mn contents than Lake Müggelsee and Lake Schwielochsee (cf. Online Resource B). Furthermore, iron oxy-hydroxides can be alternatively turned into non-reducible Fe mineral forms, including the relatively stable Fe–P mineral vivianite under anaerobic conditions (Rothe et al., 2016). Nonetheless, in all columns, Fe was mostly clearly above the detection limit in the overlying water under anoxic conditions, which indicates at least a partial dissolution of the iron oxy-hydroxides, both in the uptake and release phases, and a release of associated P.
4.2.2 Specific Lake Sediment Observations
4.2.2.1 Lake Schwielochsee
Sediments of Lake Schwielochsee showed no additional permanent fixation of phosphorus under anoxic conditions, as the P which was taken up in phase A was released back in phase B (approximately 98%). Long-term P storage in the form of vivianite was not observed, and no vivianite was found in the starting sediments. According to Rothe et al. (2015) vivianite formation takes place at elevated iron availability through a lower degree of sulfidization, which was present in lakes with S:Fe ratios < 1.1. However, in Lake Schwielochsee, the S:Fe ratio was higher than 1.1, and the sulfide concentrations from H2S in pore water at a depth greater than 2 cm were the highest (Fig. 6, up to 1.5 mg L−1) independent of the redox state at the SWI. Hydrogen sulfide competes for dissolved Fe and probably forms FeSx instead of vivianite (Heinrich et al., 2022; Roden & Edmons, 1997). This can be explained by the sulfate reduction rate in the sediments, which is controlled by the sulfate concentration in the supernatant, the organic content, the presence of the corresponding bacteria and the redox conditions (Heinrich et al., 2022).
Another special feature was the presence of chironomids in the sediments. According to Chen et al. (2015), Baranov et al. (2016) and Hupfer et al. (2019a) chironomid larvae enhance P burial in sediments, thus falsifying the amount of P retained by iron alone. Chironomid larvae built burrow-tubes in the sediments of Lake Schwielochsee. The tubes increased the sediment–water contact area, and oxic water flowed directly into the deeper depths. At these depths, normally anaerobic conditions prevail outside the tube walls; the sediments directly along the tube oxidize, and new free binding sites for P onto metal oxides are created. As the burrow walls were formed during the anoxic starting phase of the experiment, the oxygen level in the water slowly decreased, and the tubes were present at the beginning of the anoxic main phase. Thus, the sediments under anoxic conditions were able to take up higher amounts of P than those in Lake Müggelsee.
4.2.2.2 Lake Müggelsee
The net sedimentation in Lake Müggelsee was the smallest and slightly negative under anoxic conditions compared to the other three lake sediments, and the P uptake was in the same order as reported by Hupfer et al. (2019a). Furthermore, the molar Fe:P pore water ratios were < 1.2, similar to those reported by Kleeberg and Dudel (1997) and thus, the smallest compared to the other lakes. According to Grüneberg et al. (2015) Fe:P ratios < 2 are too low to prevent P release into oxic and anoxic waters.
Vivianite was present (Fig. 5) even though hydrogen sulfide concentrations in the pore water were detectable (Fig. 6). However, the S:Fe ratio of approximately 0.2 was lower than that observed in the sediments of Lake Schwielochsee, showing that there was an excess of Fe compared to S in the sediments. Friedland et al. (2021) observed that vivianite can form under natural conditions in Lake Müggelsee. According to Rothe et al. (2016) OM and Fe(III) oxy-hydroxides are the principal source materials for Fe2+ and orthophosphate in pore waters for vivianite authigenesis. However, Heinrich et al. (2021) showed that P adsorbed to iron oxyhydroxides can also be a precursor for vivianite formation using the sediments of Lake Müggelsee.
Despite vivianite formation under anoxic conditions, the sediment remained a P source under anoxic conditions. In the calcareous sediments of Lake Müggelsee the HCl-extractable P fraction (mainly Ca-bound P) did not influence the release of SRP from sediments, as the HCl-P fraction remained in the same order (12–15% considering all layers, phases, and redox states) and was much smaller than the BD-P concentration. These findings agree with those of Pettersson and Istvannovics (1988) cited in Jensen et al. (1992) showing that Ca-bound phosphorus is rather immobile.
4.2.2.3 Lake Glower See and Lake Neuendorfer See
Sediments of Lake Glower See and Lake Neuendorfer See were both P sinks in the experiment under oxic and anoxic conditions at the SWI, as net sedimentation was clearly positive (Fig. 4). This was accompanied, especially in anoxic treatments, by an increase in the P bound to Fe minerals and other metal oxides, which are not soluble under reducing conditions (NaOH-SRP, Fig. 5), including the formation of vivianite. Free hydrogen sulfide was mostly below the lowest standard, whereas SRP concentrations were higher compared to pore water measurements of Lake Schwielochsee and Lake Müggelsee, Fig. 6).
Only the sediments of Lake Glower See showed higher P uptake under anoxic than oxic conditions at low S:Fe ratios. The higher uptake under anoxic conditions was probably due to the higher degree of vivianite formation in the uppermost layer (Online Resource G).
4.3 Factors Controlling P Retention Under Ecosystem Conditions
4.3.1 Water Residence Times and Diffusion Rate
The lakes in River Spree have relatively short water residence times of a few days to weeks (Table 4). The P retention capacity of 40 Czech lakes was lower at smaller residence times, and the retention capacity in lakes with residence times < 100 days reached a maximum of 20% (Straškraba, 1996). A similarly reduced P retention owing to the short water residence time is also conceivable for the fluvial-lacustrine system Spree.
Furthermore, the chironomids in the sediments of Lake Schwielochsee not only increased the surface area (see Sect. 4.3) but probably also increased the diffusion rate of solutes due to the pumping activities of the tube-dwelling animals. Additionally, the diffusion rate depends on the porosity and tortuosity of the sediments, molecular diffusivity, bioturbation, and P concentration in the overlying water (Berg et al., 1998), which will consequently also affect P retention.
4.3.2 External P Load
A comparison of the cumulative uptake and net sedimentation in Lake Müggelsee with those of the other iron-enriched lakes in the column study demonstrated that high external P loads only resulted in high P uptake rates and high sedimentary P concentrations when binding partners, that is, Fe oxy-hydroxides, were present.
Lake Müggelsee had the smallest net sedimentation under oxic conditions and even a negative net sedimentation under anoxic conditions, whereas the other two iron-enriched lakes showed a clearly positive net sedimentation under both redox states. This is in accordance with Søndergaard et al. (1996), who found that for 32 shallow meso- to hypereutrophic Danish lakes, the sedimentary P content in the surface sediments depended on both the external P load and the sedimentary Fe content.
The high external P load of Lake Glower See compared to the other three lakes (Table. 4) can be explained by the fact that Lake Glower See receives more than 50% of its water from the polytrophic Lake Schwielochsee. The observation that a high external P load leads to an increased P retention in sediments is in accordance with Nixdorf et al. (2004) who stated that the P retention rate in Lake Neuendorfer See depends on the P content of the incoming Spree water, whereas the Fe content in the sediments was not discussed.
In the experiment, the supernatant concentrations were equated to reasonable pore water concentrations in Lake Müggelsee (Hupfer et al., 2019a) in order to determine the maximum P uptake. The P addition in the sediments simulates the P supply by settling particles in lakes, where particulate P (e.g., in the form of algae detritus) is mobilized through the degradation of organic matter. This generates high dissolved P concentrations in the SWI. Additionally, it is likely that under natural conditions, P not only binds to the sedimentary Fe in the lake (as was the case in the experiment), but also binds to particulate Fe in the inflowing Spree. This P bound to iron particles settled due to the reduced flow velocities, especially in and before the lakes (including Reservoir Spremberg), but also along the flow path of the Spree, as suggested by the TFe-SRP distribution along the Spree (Fig. 1). Firstly, this observation implies that the P loads delivered to the investigated lakes could already be reduced compared to P loads to Reservoir Bautzen without the influence of opencast mining (Fig. 1). Secondly, the retention of P in the lakes might be increased as P is already bound to settling iron oxy-hydroxides.
4.3.3 Duration of Oxic and Anoxic Periods in Lakes and Mixing Type
The experiment showed that oxygen in the overlying water additionally influenced the P uptake and release in the sediments. In lakes, the duration of oxic and anoxic phases are dependent on the mixing type. Generally, seasonal changes are much greater in shallow lakes than in deeper lakes (Yang et al., 2018). All lakes investigated are considered shallow and polymictic. Nixdorf et al. (2004) indicated that in Lake Glower See very low oxygen concentrations < 16% oxygen saturation prevailed, whereas Lake Neuendorfer See was sufficiently enriched with oxygen all year round. This means that the duration of the anoxic phase in the experiment (approximately 7 months) was much longer than observed in Lake Neuendorfer See by Nixdorf et al. (2004) so that the conditions for the formation of vivianite were not present in the lake. However, the sedimentary redoxcline can shift within the sediment and is additionally controlled by temperature, nitrate supply, and replenishment of organic material. The oxygen supply together with the higher external P load in Lake Glower See partially explains why vivianite was already present in the lake sediments in the upper 6 cm at the beginning of the experiment but not in the sediments of Lake Neuendorfer See. However, the experiment also showed that vivianite formation in the Neuendorfer See is possible when the redox conditions are suitable. In the course of further global warming due to climate change, higher temperatures and longer anoxic phases in these lakes are becoming more likely (Golosov et al., 2012; Jane et al., 2023; Pilla et al., 2020), increasing the probability of vivianite formation in Lake Neuendorfer See.
The experiment also showed that oxygen could lead to increased P uptake rates and concomitant increases in sulfate concentrations in the supernatant. We conclude that the extremely high cumulative uptake of the sediments in Lake Neuendorfer See is related to pyrite oxidation in the sediments, so that sulfate was released and new iron oxy-hydroxides were formed especially at deeper layers, providing new sorption sites for P. Goldhammer and Lenz (2020) conducted sulfur isotope analyses along the River Spree and concluded that the majority of pyrite that is formed in the sediments is also re-oxidized. Similarly, at the end of phase A in the Lake Schwielochsee sediments, the P uptake rate remained constant under oxic conditions at approximately 40 mg m−2 d−1, paired with a slight increase in sulfate concentration.
4.3.4 Other Factors Influencing the P Retention
Other factors such as pH (e.g., Roden & Edmons, 1997), temperature (e.g. Huang et al., 2011; Li et al., 2016; Liu et al., 2018; Schulz & Herzog, 2004; Zak et al., 2006), competing ions (Li et al., 2016; Zak et al., 2006) and the presence of organic matter (Chen et al., 2016; Wang et al., 2007; Zak et al., 2004) affect P retention in a lacustrine system.
The influence of pH was visible in the experiment, indicated by increased P uptake rates in the anoxic treatment of Lake Neuendorfer See when N2 was temporarily used instead of the N2-CO2- mixture (with a change in pH from 8.3 to pH 8.8). This is in concordance with Roden and Edmons (1997), who reported that higher pH values led to decreased uptake under oxic conditions, as OH− ions competitively adsorbed on Fe instead of PO43−, but may increase uptake under anoxic conditions.
The increases in NH4+-N and NO3−-N in the water column are indicators of the degradation of organic matter during the column study (Online Resource E). High concentrations of organic matter and other chemical substances with a high affiliation to Fe interfere with the aim of P retention through Fe addition. High sulfate concentrations can mediate P mobilization (Zak et al., 2006), which increased after the year ~ 2000 in the river (Zak et al., 2021) and the formation of stable organic-iron complexes could inhibit the formation of iron phosphates and iron oxides (Zak et al., 2004). As the organic matter content in the sediments was in the range of 8% in Lake Schwielochsee to 24–29% in the other lakes, it is very likely that these processes affect the sediments at different scales. In addition, nitrate prevents the dissolution of Fe(III) and leads to the oxidation of Fe(II) (Sørensen, 1982; Straub et al., 1996).
Figure 7 summarizes the factors, discussed here in Sect. 4.3, that can influence P retention in the fluvial- lacustrine system Spree, in addition to the positive effect of iron.
4.4 Implications for P Dynamics of Fluvial-Lacustrine System of River Spree
Iron has been used in different lakes worldwide to decrease TP concentrations in lake water in the short and long term. The mean incoming load in Lake Neuendorfer See from 2015 to 2020 was 101 g Fe m−2 yr−1. This is much higher than most of the applied iron amendments listed in Orihel et al. (2016) and Bakker et al. (2016). In addition, iron has been supplied continuously for decades with fluctuating amounts, whereas iron is often only added in a single dose for lake restoration. Nevertheless, positive effects on P were observed at lower doses.
Grüneberg and Kleeberg (2013) concluded from the calculation of whole-lake P-retention for a set of eight Lusatian ML that even a slight mining impact (e.g., Fe surplus) led to significantly increased P retention. Our results show that the lake sediments (especially of Lake Glower See and Lake Neuendorfer See) were generally able to retain more P in the long term, together with a clear positive correlation between Fe and P in the sediments. The molar Fe:P ratio decreased with the duration of the experiment, assuming that the amount of P that was taken up per area was distributed equally within the upper 6 cm of sediment where changes in the P concentration and P-binding forms were observed (Fig. 5). Taking the sediments of Lake Neuendorfer See in the experiment as an example and assuming the Fe content was constant, the decrease in the molar Fe:P ratio from 10.6 down to 6.6 during phase A under oxic conditions would mean that the sediments could store approximately 61% more P than were already present in the sediments. However, assuming a fictive 50% increase in P net sedimentation in the same lake (i.e., specifically from 0.8 to 1.2 g m−2 yr−1), the whole-lake P-retention would only increase from 12 to 17%. At the same time, the PLake concentration would only decrease slightly by 3 µg L−1 based on one-box model calculations (Gächter & Imboden, 1985) so that the trophic state of the lake would not be improved.
Another approach can prove that there is no marked influence of Fe based retention in Lake Neuendorfer See. We used the empirical models of Vollenweider (Vollenweider, 1976), the OECD model (Vollenweider & Kerekes, 1982) and a one-box model (Gächter & Imboden, 1985) using the net sedimentation coefficient sigma calculated after Brett and Benjamin (2008), which are valid for lakes without additional Fe input. We reproduced the measured mean PLake concentration of 0.056 mg L−1 fairly accurately from the model by Vollenweider (1976) and the one-box-model.
All three approaches make clear that iron has the potential to increase P retention in sediment, as demonstrated by the column experiments, but has no measurable effect on the PLake concentration and trophic status in Lake Neuendorfer See. There were two major reasons for this finding. Firstly, the experimental results represent an “uptake potential” under optimal conditions for P sorption (e.g., constant high SRP concentration in the overlying water). Second, this riverine lake is highly P-loaded (6.5 g m−2 yr−1) with a very short water residence time of 6.9 days, so that the water quality is controlled by external input, while internal processes (e.g., additional P sorption or release from sediment) have hardly any influence on the annual average PLake concentrations. This might differ for specific seasons, for example, when discharges are lower during summer, but this cannot be quantified with the available data.
In contrast to the studied lakes, neutral ML in east Germany have long water residence times (up to several years, e.g., Hofmann et al., 2008) and even higher Fe sediment contents than our experimental lakes (27–243 mg Fe g−1 dw; Grüneberg & Kleeberg, 2013; compared to 10–107 mg Fe g−1 dw in the studied lakes, respectively). Additionally, especially high Fe:P ratios (> 17) in the ML compared to the experimental lakes (10–11) are present, as the P load is normally lower than that in natural lakes (Grüneberg & Kleeberg, 2013). Thus, sediment P sorption in the ML largely contributes to low PLake concentrations which are mainly oligotrophic (Kleeberg & Grüneberg, 2005). Similarly, Schultze et al. (2010) concluded that eutrophication concerns in the ML of the Central German lignite mining district were of low importance for the ML.
5 Conclusion
The findings from laboratory experiments and load calculations for the interconnected lakes of the River Spree are of general importance for regions with mining influence and eutrophication concerns. The results showed that with increasing sedimentary iron concentration, net sedimentation of P increased both under oxic and anoxic conditions, and the lakes of River Spree became less susceptible to redox-driven P dynamics due to iron. While under oxic conditions P is largely adsorbed to Fe oxy-hydroxides, the formation of the Fe–P-mineral vivianite in the sediments is an important mechanism for the long-term storage of P under anoxic conditions. Hydrogen sulfide hinders vivianite authigenesis as hydrogen sulfide is the preferred binding partner for iron.
Thus, iron from lignite mining introduced to a freshwater system naturally or as a technical measure can contribute to further P fixation in the sediments, but it may not improve the trophic state of the lakes. This additional P uptake may be of little relevance to the P budget for highly loaded lakes with short water residence times typical of fluvial-lacustrine systems (10–100 d) but may significantly influence pelagic P availability and trophic state in lakes with long water residence times such as mining lakes or other adjacent lakes and reservoirs. In specific cases, P budget calculations are necessary to assess the relevance of the additional P retention and should not only be deduced from results of laboratory experiments.
Climate change and declining mining activities already cause a lower discharge in River Spree. In the short-term, the groundwater level increase will potentially lead to higher iron and sulfate concentrations in the river, while iron and sulfate loads are expected to decrease in the long-term. Therefore, future studies evaluating the effects of Fe (and SO42−) on P retention in fluvial-lacustrine systems should consider a fluctuating input of acid mine drainage products.
Data Availability
Most data generated during this study are included in this published article [and its supplementary information files]. Further data generated during the current study are available from the corresponding author on request. Further data used in this study obtained from LfU Brandenburg, SenUVK Berlin and LfULG Sachsen are available from the third parties.
References
Bakker, E. S., Van Donk, E., & Immers, A. K. (2016). Lake restoration by in-lake iron addition: A synopsis of iron impact on aquatic organisms and shallow lake ecosystems. Aquatic Ecology, 50(1), 121–135. https://doi.org/10.1007/s10452-015-9552-1
Baranov, V., Lewandowski, J., & Krause, S. (2016). Bioturbation enhances the aerobic respiration of lake sediments in warming lakes. Biology Letters, 12(8). https://doi.org/10.1098/rsbl.2016.0448
Behrendt, H. (2002). Nährstoffeinträge im Einzugsgebiet der Spree und ihre Veränderungen. In J. Köhler, J. Gelbrecht, & M. Pusch (Eds.), Die Spree: Zustand, Probleme, Entwicklungsmöglichkeiten (Limnologie aktuell Vol. 10, 1st ed., pp. 66–73). E. Schweizerbart'sche Verlagsbuchhandlung.
Berg, P., Risgaard-Petersen, N., & Rysgaard, S. (1998). Interpretation of measured concentration profiles in sediment pore water. Limnology and Oceanography, 43(7), 1500–1510. https://doi.org/10.4319/lo.1998.43.7.1500
Böckmann, C., & Päzolt, J. (2012). Regionales Nährstoffreduzierungskonzept Schwielochsee (Final report. Fachbeiträge des LUGV Nr. 125). https://lfu.brandenburg.de/sixcms/media.php/9/fb_125_schwieloch.pdf. (Accessed 08 June 2022).
Bortleson, G. C., & Lee, G. F. (1974). Phosphorus, iron, and manganese distribution in sediment cores of six Wisconsin lakes1. Limnology and Oceanography, 19(5), 794–801. https://doi.org/10.4319/lo.1974.19.5.0794
Brett, M. T., & Benjamin, M. M. (2008). A review and reassessment of lake phosphorus retention and the nutrient loading concept. Freshwater Biology, 53(1), 194–211. https://doi.org/10.1111/j.1365-2427.2007.01862.x
Busskamp, R. (2013). 5.7 Geogenic Groundwater Quality (HAD). Bundesanstalt für Gewässerkunde. https://geoportal.bafg.de/dokumente/had/57GeogenicGroundwaterQuality.pdf. Accessed 6 Jun 2023
Chen, M., Ding, S., Liu, L., Xu, D., Han, C., & Zhang, C. (2015). Iron-coupled inactivation of phosphorus in sediments by macrozoobenthos (chironomid larvae) bioturbation: Evidences from high-resolution dynamic measurements. Environmental Pollution, 204, 241–247. https://doi.org/10.1016/j.envpol.2015.04.031
Chen, M., Sun, H.-Q., & Jiang, H.-L. (2016). The addition of FeOOH binds phosphate in organic matter-rich sediments. Chemistry and Ecology, 32(5), 432–445. https://doi.org/10.1080/02757540.2016.1150455
Clayton, M. E., Liegeois, S., & Brown, J. E. (2004). Phosphorus Sequestration in Lake Sediment with Iron Mine Tailings. Soil & Sediment Contamination, 13, 421–431. https://doi.org/10.1080/10588330490500455
Cline, J. D. (1969). Spectrophotometric Determination of hydrogen sulfide in natural waters. Limnology and Oceanography, 14(3), 454–458. https://doi.org/10.4319/lo.1969.14.3.0454
Dadi, T., Rinke, K., & Friese, K. (2020). Trajectories of Sediment-Water Interactions in Reservoirs as a Result of Temperature and Oxygen Conditions. Water, 12(4), 1065. https://doi.org/10.3390/w12041065
Driescher, E. (2002). Naturräumliche Gegebenheiten und Landschaftsentwicklung. In J. Köhler, J. Gelbrecht, & M. Pusch (Eds.), Die Spree: Zustand, Probleme, Entwicklungsmöglichkeiten (Limnologie aktuell Vol. 10, 1st ed., pp. 1–25). E. Schweizerbart'sche Verlagsbuchhandlung.
Friedland, G., Grüneberg, B., & Hupfer, M. (2021). Geochemical signatures of lignite mining products in sediments downstream a fluvial-lacustrine system. Science of The Total Environment, 760, 143942. https://doi.org/10.1016/j.scitotenv.2020.143942
Friese, K., Wendt-Potthoff, K., Zachmann, D. W., Fauville, A., Mayer, B., & Veizer, J. (1998). Biogeochemistry of iron and sulfur in sediments of an acidic mining lake in Lusatia, Germany. Water, Air, & Soil Pollution, 108(3–4), 231–247. https://doi.org/10.1023/A:1005195617195
Gabriel, O., Balla, D., Kalettka, T., & Maassen, S. (2008). Sink or source? - The effect of hydrology on phosphorus release in the cultivated riverine wetland Spreewald (Germany). Water Science and Technology, 58(9), 1813–1822. https://doi.org/10.2166/wst.2008.564
Gächter, R., & Imboden, D. (1985). Lake Restoration. In W. Stumm (Ed.), Chemical processes in Lakes (pp. 363–388). John Wiley & Sons.
Goldhammer, T., & Lenz, J. (2020). Isotope insights into sulfate loads and sulfur cycling in a heavily polluted river network. EGU General Assembly 2020, Online, 4–8 May 2020, EGU2020-14997. https://doi.org/10.5194/egusphere-egu2020-14997.
Golosov, S., Terzhevik, A., Zverev, I., Kirillin, G., & Engelhardt, C. (2012). Climate change impact on thermal and oxygen regime of shallow lakes. Tellus A: Dynamic Meteorology and Oceanography, 64(1), 17264. https://doi.org/10.3402/tellusa.v64i0.17264
Golterman, H. L. (1988). The calcium- and iron bound phosphate phase diagram. Hydrobiologia, 159(2), 149–151. https://doi.org/10.1007/BF00014722
Golterman, H. L. (1995). The role of the ironhydroxide-phosphate-sulphide system in the phosphate exchange between sediments and overlying water. Hydrobiologia, 297(1), 43–54. https://doi.org/10.1007/BF00033500
Grazulis, S., Daskevic, A., Merkys, A., Chateigner, D., Lutterotti, L., Quiros, M., Serebryanaya, N. R., Moeck, P., Downs, R. T., & Le Bail, A. (2012). Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Research, 40(D1), D420–D427. https://doi.org/10.1093/nar/gkr900
Grüneberg, B., Dadi, T., Lindim, C., & Fischer, H. (2015). Effects of nitrogen and phosphorus load reduction on benthic phosphorus release in a riverine lake. Biogeochemistry, 123, 185–202. https://doi.org/10.1007/s10533-014-0062-3
Grüneberg, B., & Kleeberg, A. (2013). Phosphorous in Acidic Mining Lakes: Importance and Biogeochemical Cycling. In W. Geller, M. Schultze, R. Kleinmann, & C. Wolkersdorfer (Eds.), Acidic Pit Lakes - The Legacy of Coal and Metal Surface Mines. (1st. ed., pp. 62–75). Springer. https://doi.org/10.1007/978-3-642-29384-9
Hamilton, S. K., Bruesewitz, D. A., Horst, G. P., Weed, D. B., & Sarnelle, O. (2009). Biogenic calcite–phosphorus precipitation as a negative feedback to lake eutrophication. Canadian Journal of Fisheries and Aquatic Sciences. https://doi.org/10.1139/F09-003
Hansen, J., Reitzel, K., Jensen, H. S., & Andersen, F. Ø. (2003). Effects of aluminum, iron, oxygen and nitrate additions on phosphorus release from the sediment of a Danish softwater lake. Hydrobiologia, 492(1), 139–149. https://doi.org/10.1023/A:1024826131327
Heidenreich, M., & Kleeberg, A. (2003). Phosphorus-binding in iron-rich sediments of a shallow Reservoir: Spatial characterization based on sonar data. Hydrobiologia, 506–509, 147–153. https://doi.org/10.1023/B:HYDR.0000008621.44435.23
Heinrich, L., Dietel, J., & Hupfer, M. (2022). Sulphate reduction determines the long-term effect of iron amendments on phosphorus retention in lake sediments. Journal of Soils and Sediments. https://doi.org/10.1007/s11368-021-03099-3
Heinrich, L., Rothe, M., Braun, B., & Hupfer, M. (2021). Transformation of redox-sensitive to redox-stable iron-bound phosphorus in anoxic lake sediments under laboratory conditions. Water Research, 189, 116609. https://doi.org/10.1016/j.watres.2020.116609
Herzsprung, P., Schultze, M., Hupfer, M., Boehrer, B., Tümpling, W. V., Duffek, A., Van der Veen, A., & Friese, K. (2010). Flood effects on phosphorus immobilisation in a river water filled pit lake—Case study Lake Goitsche (Germany). Limnologica, 40(2), 182-190.https://doi.org/10.1016/j.limno.2009.11.007.
Hofmann, H., Knöller, K., & Lessmann, D. (2008). Mining lakes as groundwater-dominated hydrological systems: Assessment of the water balance of Mining Lake Plessa 117 (Lusatia, Germany) using stable isotopes. Hydrological Processes, 22(23), 4620–4627. https://doi.org/10.1002/hyp.7071
Huang, L., Fu, L., Jin, C., Gielen, G., Lin, X., Wang, H., & Zhang, Y. (2011). Effect of temperature on phosphorus sorption to sediments from shallow eutrophic lakes. Ecological Engineering, 37(10), 1515–1522. https://doi.org/10.1016/j.ecoleng.2011.05.006
Hupfer, M., Gächter, R., & Giovanoli, R. (1995). Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquatic Sciences, 57(4), 305–324. https://doi.org/10.1007/bf00878395
Hupfer, M., Gelbrecht, J., Köhler, J., Ackermann, G., & Schulz, M. (2002). Nährstoffdynamik. In J. Köhler, J. Gelbrecht, & M. Pusch (Eds.), Die Spree: Zustand, Probleme, Entwicklungsmöglichkeiten (Limnologie aktuell Vol. 10, 1st ed., pp. 100–110). E. Schweizerbart'sche Verlagsbuchhandlung.
Hupfer, M., Jordan, S., Herzog, C., Ebeling, C., Ladwig, R., Rothe, M., & Lewandowski, J. (2019a). Chironomid larvae enhance phosphorus burial in lake sediments: Insights from long-term and short-term experiments. Science of the Total Environment, 663, 254–264. https://doi.org/10.1016/j.scitotenv.2019.01.274
Hupfer, M., & Lewandowski, J. (2008). Oxygen Controls the Phosphorus Release from Lake Sediments – a Long-Lasting Paradigm in Limnology. International Review of Hydrobiology, 93(4–5), 415–432. https://doi.org/10.1002/iroh.200711054
Hupfer, M., Reitzel, K., & Grüneberg, B. (2019b). Methods for measuring internal loading. In A. D. Steinman & B. M. Spears (Eds.), Internal phosphorus loading in lakes : Causes, case studies, and management (pp. 15–43). J. Ross Publ.
Hupfer, M., Reitzel, K., Kleeberg, A., & Lewandowski, J. (2016). Long-term efficiency of lake restoration by chemical phosphorus precipitation: Scenario analysis with a phosphorus balance model. Water Research, 97, 153–161. https://doi.org/10.1016/j.watres.2015.06.052
Hupfer, M., & Scharf, B. W. (2014). Seentherapie: Interne Maßnahmen zur Verminderung der Phosphorkonzentration. In W. Calmano, M. Hupfer, H. Fischer, & H. Klapper (Eds.), Handbuch Angewandte Limnologie: Grundlagen - Gewässerbelastung - Restaurierung - Aquatische Ökotoxikologie - Bewertung - Gewässerschutz (1st ed., pp. 1–67). Wiley‐VCH Verlag. https://doi.org/10.1002/9783527678488.hbal2002001
IGB (Leibniz Institute of Freshwater Ecology and Inland Fisheries Berlin). (2021). Müggelsee nutrients 2015–2019. https://doi.org/10.18728/igb-fred-573.2
Jane, S. F., Mincer, J. L., Lau, M. P., Lewis, A. S. L., Stetler, J. T., & Rose, K. C. (2023). Longer duration of seasonal stratification contributes to widespread increases in lake hypoxia and anoxia. Global Change Biology, 29(4), 1009–1023. https://doi.org/10.1111/gcb.16525
Jensen, H. S., Kristensen, P., Jeppesen, E., & Skytthe, A. (1992). Iron:Phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiologia, 235(236), 731–743. https://doi.org/10.1007/BF00026261
Katsev, S. (2016). Phosphorus Effluxes from Lake Sediments. In R. Lal & B. A. Stewart (Eds.), Soil Phosphorus (1st ed., pp. 115–131). CRC Press. https://doi.org/10.1201/9781315372327-7
Kim, J., Li, W., Philips, B. L., & Grey, C. P. (2011). Phosphate adsorption on the iron oxyhydroxides goethite (α-FeOOH), akaganeite (β-FeOOH), and lepidocrocite (γ-FeOOH): A 31P NMR Study. Energy & Environmental Science, 4(10), 4298–4305. https://doi.org/10.1039/C1EE02093E
Kleeberg, A., & Dudel, G. E. (1997). Changes in extent of phosphorus release in a shallow lake (Lake Groβer Müggelsee; Germany, Berlin) due to climatic factors and load. Marine Geology, 139(1), 61–75. https://doi.org/10.1016/S0025-3227(96)00099-0
Kleeberg, A., & Grüneberg, B. (2005). Phosphorus mobility in sediments of acid mining lakes, Lusatia, Germany. Ecological Engineering, 24(1), 89–100. https://doi.org/10.1016/j.ecoleng.2004.12.010
Kleeberg, A., Herzog, C., & Hupfer, M. (2013). Redox sensitivity of iron in phosphorus binding does not impede lake restoration. Water Research, 47(3), 1491–1502. https://doi.org/10.1016/j.watres.2012.12.014
Kleeberg, A., Herzog, C., Jordan, S., & Hupfer, M. (2010). What drives the evolution of the sedimentary phosphorus cycle? Limnologica, 40(2), 102–113. https://doi.org/10.1016/j.limno.2009.11.001
Köhler, J. (1994). Origin and succession of phytoplankton in a river-lake system (Spree, Germany). Hydrobiologia, 289(1), 73–83. https://doi.org/10.1007/bf00007410
Köhler, J., Petzoldt, T., Köher, A., Kruspe, U., Täuscher, H., & Mischke, U. (2002). Das Phytoplankton im Spreesystem. In J. Köhler, J. Gelbrecht, & M. Pusch (Eds.), Die Spree: Zustand, Probleme, Entwicklungsmöglichkeiten (Limnologie aktuell Vol. 10, 1st ed., pp. 144–153). E. Schweizerbart'sche Verlagsbuchhandlung.
Koschel, R., Benndorf, J., Proft, G., & Recknagel, F. (1983). Calcite precipitation a a natural control mechnism of eutrophication. Archiv Für Hydrobiologie, 98(3), 380–408.
Kozerski, H.-P., & Kleeberg, A. (1998). The Sediments and Benthic-Pelagic Exchange in the Shallow Lake Müggelsee (Berlin, Germany). International Review of Hydrobiology, 83(1), 77–112. https://doi.org/10.1002/iroh.19980830109
LAVG (Landesamt für Arbeitsschutz, Verbraucherschutz und Gesundheit). (2021). Badegewässerprofil Glower See. https://badestellen.brandenburg.de/documents/51161/63385/077.pdf. (Accessed 08 June 2022).
LAVG (Landesamt für Arbeitsschutz, V. u. G. (2023). EU-Badestelle Hohenbrück Neuendorfer See. https://www.dahme-spreewald.info/media_fast/595/Steckbrief_Neuendorfer_See_Hohenbr%C3%BCck.pdf. (Accessed 09 June 2023).
LAWA (Länderarbeitsgemeinschaft Wasser). (1999). Gewässerbewertung stehende Gewässer - Vorläufige Richtlinie für eine Erstbewertung von natürlich entstandenen Seen nach trophischen Kriterien (p. 75). Kulturbuch-Verlag.
LAWA (Länderarbeitsgemeinschaft Wasser). (2003). Ermittlung von Stoff-Frachten in Fließgewässern - Probenahmestrategien und Berechnungsverfahren (p. 76). Kulturbuch-Verlag.
LfU (Landesamt für Umwelt Brandenburg). (2017a). Steckbrief Seen EU-Wasserrahmenrichtlinie Neuendorfer See. Retrieved from. https://lfu.brandenburg.de/daten/w/seen/800015827133.pdf. (Accessed 08 June 2022).
LfU (Landesamt für Umwelt Brandenburg). (2017b). Steckbrief Seen EU-Wasserrahmenrichtlinie Schwielochsee. Retrieved from. https://lfu.brandenburg.de/daten/w/seen/80001582739.pdf. (Accessed 08 June 2022).
LfU (Landesamt für Umwelt Brandenburg). (2019). Water chemistry data: Sulfate and Total Iron concentrations (2015–2019) (retrieved 19.12.2019).
LfU (Landesamt für Umwelt Brandenburg). (2021a). Discharge data (2015–2019) Leibsch Spreewehr UP (retrieved 08.11.2021a).
LfU (Landesamt für Umwelt Brandenburg). (2021b). Water chemistry data: TP and SRP concentrations (2015–2019) (retrieved 18.01.2021b).
LfU (Landesamt für Umwelt Brandenburg). (2023). Water chemistry data: Sulfate, SRP, TP concentrations in Lake Schwielochsee, euphotic zone (retrieved 08.02.2023).
LfULG (Landesamt für Umwelt, Landwirtschaft und Geologie Sachsen),. (2019). Water chemistry data: Sulfate and Total Iron concentrations (2015–2019). https://www.umwelt.sachsen.de/umwelt/infosysteme/ida/pages/map/default/index.xhtml. (Accessed 10 Jan 2020).
LfULG (Landesamt für Umwelt, Landwirtschaft und Geologie Sachsen),. (2022). Water chemistry data: SRP concentrations (2015–2019). https://www.umwelt.sachsen.de/umwelt/infosysteme/ida/pages/map/default/index.xhtml. (Accessed 21 Jan 2022).
Li, M., Liu, J., Xu, Y., & Qian, G. (2016). Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environmental Reviews, 24(3), 319–332. https://doi.org/10.1139/er-2015-0080
Liu, Q., Ding, S., Chen, X., Sun, Q., Chen, M., & Zhang, C. (2018). Effects of temperature on phosphorus mobilization in sediments in microcosm experiment and in the field. Applied Geochemistry, 88, 158–166. https://doi.org/10.1016/j.apgeochem.2017.07.018
Markovic, S., Liang, A., Watson, S. B., Guo, J., Mugalingam, S., Arhonditsis, G., Morley, A., & Dittrich, M. (2019). Biogeochemical mechanisms controlling phosphorus diagenesis and internal loading in a remediated hard water eutrophic embayment. Chemical Geology, 514, 122–137. https://doi.org/10.1016/j.chemgeo.2019.03.031
Moosmann, L., Gächter, R., Müller, B., & Wüest, A. (2006). Is phosphorus retention in autochthonous lake sediments controlled by oxygen or phosphorus? Limnology and Oceanography, 51(1part2), 763–771. https://doi.org/10.4319/lo.2006.51.1_part_2.0763.
Mortimer, C. H. (1941). The Exchange of Dissolved Substances Between Mud and Water in Lakes. The Journal of Ecology, 29(2), 280–329. https://doi.org/10.2307/2256395
Nixdorf, B., Hemm, M., Hoffmann, A., & Richter, P. (2004). Dokumentation von Zustand und Entwicklung der wichtigsten Seen Deutschlands - Teil 5 Brandenburg (Final report of F&E Vorhaben FKZ 299 24 274). https://www-docs.b-tu.de/fg-gewaesserschutz/public/projekte/uba_2/05_brandenburg.pdf. (Accessed 08 June 2022).
Nützmann, G., Wolter, C., Venohr, M., & Pusch, M. (2011). Historical Patterns of Anthropogenic Impacts on Freshwaters in the Berlin-Brandenburg Region. Die Erde, 142(1–2), 41–64. https://doi.org/10.12854/erde.v142i1-2.42.
Orihel, D. M., Baulch, H. M., Casson, N. J., North, R. L., Parsons, C. T., Seckar, D. C. M., & Venkiteswaran, J. J. (2017). Internal phosphorus loading in Canadian fresh waters: A critical review and data analysis. Canadian Journal of Fisheries and Aquatic Sciences, 74, 2005–2029. https://doi.org/10.1139/cjfas-2016-0500
Orihel, D. M., Schindler, D. W., Ballard, N. C., Wilson, L. R., & Vinebrooke, R. D. (2016). Experimental iron amendment suppresses toxic cyanobacteria in a hypereutrophic lake. Ecological Applications, 26(5), 1517–1534. https://doi.org/10.1890/15-1928
Pettersson, K., & Istvannovics, V. (1988). Sediment phosphorus in Lake Balaton–forms and mobility. Archiv Für Hydrobiologie, 30, 25–41.
Pilla, R. M., Williamson, C. E., Adamovich, B. V., Adrian, R., Anneville, O., Chandra, S., Colom-Montero, W., Devlin, S. P., Dix, M. A., Dokulil, M. T., Gaiser, E. E., Girdner, S. F., Hambright, K. D., Hamilton, D. P., Havens, K., Hessen, D. O., Higgins, S. N., Huttula, T. H., Huuskonen, H., & Zadereev, E. (2020). Deeper waters are changing less consistently than surface waters in a global analysis of 102 lakes. Scientific Reports, 10(1), 20514. https://doi.org/10.1038/s41598-020-76873-x
Psenner, R., Pucsko, R., & Sager, M. (1984). Fractionation of Organic and Inorganic Phosphorus Compounds in Lake Sediments, An Attempt to Characterize Ecologically Important Fractions. Archiv Für Hydrobiologie, 70, 111–155.
R Core Team. (2017). R: A language and environment for statistical computing. In R Foundation for Statistical Computing (Ed.). Vienna, Austria. https://www.R-project.org/
Rickard, D. (2012). Chapter 3 - Sedimentary Iron Biogeochemistry. In D. Rickard (Ed.), Developments in Sedimentology (vol. 65, pp. 85–119). Elsevier. https://doi.org/10.1016/B978-0-444-52989-3.00003-9.
Roden, E. E., & Edmons, J. W. (1997). Phosphate mobilization in iron-rhich anaerobic sediments: Microbial Fe(III) oxide reduction versus iron-sulfide formation. Archiv Für Hydrobiologie, 139(3), 347–378. https://doi.org/10.1127/archiv-hydrobiol/139/1997/347
Rothe, M., Kleeberg, A., Grüneberg, B., Friese, K., Pérez-Mayo, M., & Hupfer, M. (2015). Sedimentary Sulphur: Iron Ratio Indicates Vivianite Occurrence: A Study from Two Contrasting Freshwater Systems. PLoS ONE, 11, 1–18. https://doi.org/10.1371/journal.pone.0143737
Rothe, M., Kleeberg, A., & Hupfer, M. (2016). The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments. Earth-Science Reviews, 158, 51–64. https://doi.org/10.1016/j.earscirev.2016.04.008
Schindler, D. W. (2006). Recent advances in the understanding and management of eutrophication. Limnology and Oceanography, 51, 356–363. https://doi.org/10.4319/lo.2006.51.1_part_2.0356
Schmidt, S. R., Lischeid, G., Hintze, T., & Adrian, R. (2019). Disentangling limnological processes in the time-frequency domain. Limnology and Oceanography, 64(2), 423–440. https://doi.org/10.1002/lno.11049
Schultze, M., Pokrandt, K.-H., & Hille, W. (2010). Pit lakes of the Central German lignite mining district: Creation, morphometry and water quality aspects. Limnologica, 40(2), 148–155. https://doi.org/10.1016/j.limno.2009.11.006
Schulz, M., & Herzog, C. (2004). The influence of sorption processes on the phosphorus mass balance in a eutrophic German lowland river. Water, Air, & Soil Pollution, 155, 291–301. https://doi.org/10.1023/B:WATE.0000026535.27164.56
SenUVK (Senate Department for the Environment, Transport and Climate Protection). (2020). Water chemistry data: Sulfate and Total Iron concentrations (2015–2019) (retrieved 09.06.2020).
SenUVK (Senate Department for the Environment, Transport and Climate Protection). (2021). Water chemistry data: SRP concentrations (2015–2019) (retrieved 15.09.2021).
Shatwell, T., & Köhler, J. (2019). Decreased nitrogen loading controls summer cyanobacterial blooms without promoting nitrogen-fixing taxa: Long-term response of a shallow lake. Limnology and Oceanography, 64(S1), S166–S178. https://doi.org/10.1002/lno.11002
Shotbolt, L. (2010). Pore water sampling from lake and estuary sediments using Rhizon samplers. Journal of Paleolimnology, 44(2), 695–700. https://doi.org/10.1007/s10933-008-9301-8
Sibrell, P. L., Montgomery, G. A., Ritenour, K. L., & Tucker, T. W. (2009). Removal of phosphorus from agricultural wastewaters using adsorption media prepared from acid mine drainage sludge. Water Research, 43(8), 2240–2250. https://doi.org/10.1016/j.watres.2009.02.010
Simmons, J. A. (2010). Phosphorus Removal by Sediment in Streams Contaminated with Acid Mine Drainage. Water, Air, & Soil Pollution, 209(1), 123–132. https://doi.org/10.1007/s11270-009-0185-7
Søndergaard, M., Windolf, J., & Jeppesen, E. (1996). Phosphorus fractions and profiles in the sediment of shallow Danish lakes as related to phosphorus load, sediment composition and lake chemistry. Water Research, 30(4), 992–1002. https://doi.org/10.1016/0043-1354(95)00251-0
Sørensen, J. (1982). Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Applied and Environmental Microbiology, 43(2), 319–324. https://doi.org/10.1128/aem.43.2.319-324.1982
Spikjerman, E. (2008). Phosphorus limitation of algae living in iron-rich, acidic lakes. Aquatic Microbial Ecology, 53, 201–210. https://doi.org/10.3354/ame01244
SenS (Senatsverwaltung für Stadtentwicklung) (2005). Gewässeratlas von Berlin - Von der Gewässervermessung zum Gewässeratlas von Berlin mit hydrographischem Informationssystem. https://www.berlin.de/sen/uvk/_assets/umwelt/wasser-und-geologie/publikationen-und-merkblaetter/wasseratlas.pdf. Accessed 6 Jun 2023
Straškraba, M. (1996). Lake and reservoir management. SIL Proceedings, 1922–2010, 26(1), 193–209. https://doi.org/10.1080/03680770.1995.11900703.
Straub, K. L., Benz, M., Schink, B., & Widdel, F. (1996). Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Applied and Environmental Microbiology, 62(4), 1458–1460. https://doi.org/10.1128/aem.62.4.1458-1460.1996
Tang, Z., & Nairn, R. W. (2021). Mine Drainage Residual Additions to Lake Sediments Alter Phosphorus and Trace Metal Distributions. Water, Air, & Soil Pollution, 232(2), 52. https://doi.org/10.1007/s11270-021-05016-3
Tate, C. M., Broshears, R. E., & McKnight, D. M. (1995). Phosphate dynamics in an acidic mountain stream: Interactions involving algal uptake, sorption by iron oxide, and photoreduction. Limnology and Oceanography, 40(5), 938–946. https://doi.org/10.4319/lo.1995.40.5.0938
Vollenweider, R. (1976). Advances in defining critical loading levels for phosphorus in lake eutrophication. Memorie Dell’ Istituto Italiano Di Idrobiologia, 33, 53–83.
Vollenweider, R., & Kerekes, J. (1982). Eutrophication of waters. Monitoring, assessment and control (p. 154). Paris: OECD.
Wang, S., Jin, X., Zhao, H., Zhou, X., & Wu, F. (2007). Effect of organic matter on the sorption of dissolved organic and inorganic phosphorus in lake sediments. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 297(1), 154–162. https://doi.org/10.1016/j.colsurfa.2006.10.040
Wanner, S. C., Ockenfeld, K., Brunke, M., Fischer, H., & Pusch, M. (2002). The distribution and turnover of benthic organic matter in a lowland river: Influence of hydrology, seston load and impoundment. River Research and Applications, 18(2), 107–122. https://doi.org/10.1002/rra.636
Wei, X., Viadero, R. C., & Bhojappa, S. (2008). Phosphorus removal by acid mine drainage sludge from secondary effluents of municipal wastewater treatment plants. Water Research, 42(13), 3275–3284. https://doi.org/10.1016/j.watres.2008.04.005
Yang, Y., Wang, Y., Zhang, Z., Wang, W., Ren, X., Gao, Y., Liu, S., & Lee, X. (2018). Diurnal and Seasonal Variations of Thermal Stratification and Vertical Mixing in a Shallow Fresh Water Lake. Journal of Meteorological Research, 32(2), 219–232. https://doi.org/10.1007/s13351-018-7099-5
Zak, D., Gelbrecht, J., & Steinberg, C. E. W. (2004). Phosphorus Retention at the Redox Interface of Peatlands Adjacent to Surface Waters in Northeast Germany. Biogeochemistry, 70(3), 357–368. https://doi.org/10.1007/s10533-003-0895-7
Zak, D., Hupfer, M., Cabezas, A., Jurasinski, G., Audet, J., Kleeberg, A., McInnes, R., Kristiansen, S. M., Petersen, R. J., Liu, H., & Goldhammer, T. (2021). Sulphate in freshwater ecosystems: A review of sources, biogeochemical cycles, ecotoxicological effects and bioremediation. Earth-Science Reviews, 212, 103446. https://doi.org/10.1016/j.earscirev.2020.103446
Zak, D., Kleeberg, A., & Hupfer, M. (2006). Sulphate-mediated phosphorus mobilization in riverine sediments at increasing sulphate concentration, River Spree, NE Germany. Biogeochemistry, 80, 109–119. https://doi.org/10.1007/s10533-006-0003-x
Acknowledgements
We are grateful to our colleagues of Department Ecohydrology and Biogeochemistry at IGB Berlin, for conducting the CFA, ICP-OES, TP and AAS analyses. We thank Grit Siegert for C/N/S analysis of the sediments. We thank Marvin Bethke and Sylvia Jordan for help with the installation of the column experiment. We also thank the Landesamt für Umwelt Brandenburg (LfU) and Senate Department of the Environment, Transport and Climate Protection in Berlin for making water chemistry data and discharge data for the River Spree available upon request and the Landesamt of Saxony for open access. We further thank the LfU for providing lake morphometric data of Lake Neuendorfer See and Lake Schwielochsee We finally thank “editage” for English language editing and two anonymous reviewers for their constructive feedback on a former version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The research is part of the Graduate Research School ‘Signatures of severely disturbed landscapes—case study mining landscapes’ of the BTU Cottbus-Senftenberg. The project is funded by the Leibniz Institute of Freshwater Ecology and Inland Fisheries.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and statistical analysis were performed by Giulia Kommana. Calculation of Loads were done by Giulia Kommana and Björn Grüneberg. The first draft of the manuscript was written by Giulia Kommana and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kommana, G., Grüneberg, B. & Hupfer, M. Iron from Lignite Mining Increases Phosphorus Fixation in Sediments, but Does Not Affect Trophic States of Lakes Along River Spree (Germany). Water Air Soil Pollut 234, 454 (2023). https://doi.org/10.1007/s11270-023-06441-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06441-2