Abstract
Forest decline, which occurs in mountainous regions in many countries, may result from the effects of acid fog, ozone, or deposition of other pollutants. We observed wet deposition of air pollutants at different altitudes on Mt. Oyama, situated southwest of Tokyo, for 1994 until 2019. During this period, the domestic atmospheric environment was improved in Japan. The average concentration of air pollutants around all the sampling sites on Mt. Oyama decreased by 63.01%, 32.08%, 8.80%, and 39.73% for NH3, HCl, HNO3, and SO2, respectively. The volume weighted mean (VWM) pH values showed an increasing trend for bulk deposition (+0.70% y-1), fog water (+2.58% y-1), and throughfall (+2.60% y-1). Stemflow also increased (cedar, +1.17% y-1; fir, +0.82% y-1), although it included organic acids dissolved from the stem and it primarily had a low VWM pH value. The overall pH value of the fog water increased at the site, although acidic fog was still observed. Comparing fog water between winter and summer, a significant increase in pH occurred in summer, whereas no change occurred in winter. In summer, the sources of pollutants at Mt. Oyama are mainly from mainland Japan, and the frequency of low-pH acidic fog decreased annually. In winter, Mt. Oyama was affected by transboundary pollution, and acid fog occasionally occurred. Improvements in the domestic atmospheric environment and control of transboundary pollution will provide better conditions for mountain ecology that are free from acidic pollutants.
Similar content being viewed by others
1 Introduction
Forests cover 32% of the world's terrestrial ecosystems and provide many ecosystem services, including hydrological regulation, soil conservation, climate change mitigation, and air quality regulation (Millar & Stephenson, 2015; Nowak et al., 2008). However, due to the rapid economic growth and inadequate pollution control, human emissions continue to increase, resulting in air pollution. Air pollution and acid deposition stem from the same anthropogenic sources. The deposition of nitrogen (N) sometimes enhances tree growth rates (Horn et al., 2018; Maes et al., 2019), whereas sulfur dioxide, nitrogen oxides, and ozone adversely affect tree health (Paoletti et al., 2010; Ren et al., 2011).
Forest decline has been observed over the past several decades, not only in Europe and North America (Menz & Seip, 2004; Pitelka & Raynal, 1989; Schutt & Cowling, 1985) but also in East Asia, including Japan (Sekiguchi et al., 1986), China (Bian & Yu, 1992), Korea (Kim & Lee, 1993), and Russia (Kiseleva, 1996). The decline is caused mainly by air pollutants through dry and wet deposition (Aikawa et al., 2006; Anderson et al., 1999; Bussotti & Ferretti, 1998; Igawa et al., 2002; Johnson & Siccama, 1983; Takahashi et al., 2020; Vong et al., 1991).
In the 1970s, fir trees (Abies firma), a typical evergreen coniferous tree in the middle temperate zone of Japan, were wilted and declined on the southeastern slopes of Mt. Oyama in the Tanzawa mountain range, 50 km west of Tokyo (Suzuki, 1992). In the 1980s, a decline in Japanese beech (Fagus crenata), a typical temperate deciduous broad-leaved tree in Japan, became apparent in the ridgeline between Mt. Tonodake and Hirugatake, and around the peak of Mt. Hinokiboramaru (Kohno, 2017) in the Tanzawa Mountains. This has been noted to be caused by the above air pollutants through dry deposition (e.g. ozone) (Kohno et al., 2005; Takeda & Aihara, 2007; Uno et al., 1996), wet deposition (e.g. acid fog) (Igawa et al., 2002; Igawa, Kase, et al., 2002; Shigihara et al., 2008, 2009). Acid deposition often causes soil acidification leading to forest decline, but no soil acidification was observed in the declining site of the Tanzawa Mountains (Ohse et al., 2003). In addition, recent forest decline may have occurred in conjunction with climate change, such as drought, heat, and extreme climate events (Takahashi et al., 2020). However, air pollution is the predominant cause of the decline and monitoring air pollution is important to determine the causes. Trees in forests can be considered as the bio-monitor of air pollution by observing their health levels (Al-Alawi et al., 2007; Al-Alawi & Mandiwana, 2007; Batarseh et al., 2008).
A 30-year observation of soils in the southern China in Asia showed that soil acidification is caused by atmospheric pollution and that the concentration of acid deposition (sulfur) in the soil is higher where the source of pollution is close (Dai et al., 1998). On the other hand, in North America and Europe, long-term monitoring of precipitation, soil, and surface water chemistry has shown that forest health and water quality have been restored by reducing sulfur dioxide and nitrogen oxide emissions and associated sulfate and nitrate deposition (Likens et al., 1996; Pannatier et al., 2011; Watmough et al., 2016; Wright & Hons, 2005). The obvious example of a striking trend is the recovery in the growth of red spruce (Picea rubens Sarg.) in the northeastern United States where the deposition of sulfate and nitrate in the forest decreases (Kosiba et al., 2018). However, Lawrence et al. (2019) reported that even as the atmospheric deposition of nitrogen decreased, watershed nitrogen export in the western Adirondack region of New York State has not decreased, and toxic aluminum and nitrate continue to be released from the soil into streams. The recovery of forest productivity and water quality depends on each ecosystem, and considerable uncertainty remains regarding the controlling factors. Therefore, there is a need for long-term monitoring of precipitation, soil, and surface water chemistry in the different types of forest ecosystems in each region.
There were many reports on the air pollution in mountainous areas, but most of their observation periods were short (Abe et al., 2022; Aikawa et al., 2006; Chiwa et al., 2003; Igawa et al., 1998; Igawa, Matsumura, & Okochi, 2002), which may be due to factors such as difficulty of sampling for field observations, high maintenance costs, time-consuming, and laborious. Short-term observations can only indicate changes in the impact on forest during this observation period, while the decline of trees was formed by long term effects. Some papers reported long term observational studies, but the observations were limited to wet deposition (Avila, 1996; Igawa et al., 2015; Tago et al., 2006; Watanabe et al., 2022) or dry deposition (Phu Nguyen et al., 2019; Takeuchi et al., 2004; Tanimoto, 2009) or limited to observations at one elevation, while decline of trees would not be restricted to one elevation or caused by one effect of wet or dry deposition alone. In conclusion, short-term observations cannot sufficiently explain the underlying causes of the tree decline, and the cause of the tree decline is likewise not clarified well by relying on the observation at a single site or a single observation program under a long-term observation.
In this study, we aimed to assess long-term (26 y) trends at different altitudes in wet and dry deposition on Mt. Oyama near the Tokyo metropolitan area. The altitude dependence of the concentrations and deposition of air pollutants was also determined, and transboundary air pollution was examined using backward trajectory analyses.
2 Experimental
2.1 Observation sites
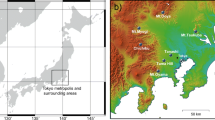
The Tanzawa mountains are a mountain range of approximately 40 kha in the Kanto District of Japan, which is located approximately 50 km west–southwest of Tokyo. They were registered as Kanagawa Prefectural Natural Park in 1960 and as a quasi-national park in 1965. The highest peak is Mt. Hirugatake (1673 m a.s.l.), and the other main peaks are Hinokiboramaru (1601 m a.s.l., 4th height), Tanzawa-san (1567 m a.s.l., 7th height), and Tonodake (1491 m a.s.l., 12th height). Figure 1 shows the sampling points on the southern and southeastern slopes of Mt. Oyama (1252 m a.s.l., 36th height, 35°26'N; 139°13'E), which is an independent mountain at the southeastern edge of the Tanzawa Mountains. The bulk deposition, throughfall, and stemflow of Japanese cedar and fir, and fog water were collected monthly at 400, 680, 890, 940, 1062, and 1252 m a.s.l. (the summit), on Mt. Oyama from 1994 to 2019.
2.2 Collection and observation methods
Fog water samples were collected using a homemade passive string fog collector (PFC) with a 60 cm disc at its top to prevent rainwater intrusion (Igawa et al., 2015) at sampling sites of 680, 940, 1062, and 1252 m a.s.l. on Mt. Oyama. Briefly, fog water collided with a cylindrical net consisting of 72 Teflon tubes (outer diameter:1 mm) and was captured on them. It fell owing to its own weight and was filtered through a membrane filter (A100A047A: Toyo Filter Paper Co., Ltd.) with a pore size of 1.0 μm. It was then stored in a 5 L brown propylene bottle for light shielding to prevent water quality deterioration.
The bulk deposition and throughfall collector consisted of a funnel with a diameter of 80 mm, a filter holder including a membrane filter with a pore size of 1.0 μm, and a 5 L brown polypropylene bottle. Each part was connected to a silicon tube. These collectors were placed in open areas to collect bulk deposition at altitudes of 400, 940, and 1252 m a.s.l., and under cedar and fir trees to collect throughfall at altitudes of 680, 890, and 1252 m a.s.l., respectively. Stemflow of cedar and fir trees was collected using the gauze trap method (Sassa et al., 1990) at 890 m a.s.l.
Gases (SO2, HNO3, HCl, NH3, and O3) were also observed using the passive sampling method (Yanagisawa & Nishimura, 1980) with a filter badge for SO2 measurement (07810160, Advantec, Co.) (Igawa et al., 2007) at four altitudes of 400, 680, 890, and 1252 m a.s.l. on Mt. Oyama. The capture area of the collector was 42 × 30 mm2(L × B) Acid gases were collected by impregnating a cellulose filter with a solution of 1% Na2CO3, 1% glycerol, and NH3 gas and a solution of 5% H3PO4 and 5% glycerol. For O3 measurement, a filter paper was impregnated with a solution of 0.1% Na2NO2, 0.2% Na2CO3, and 1% ethylene glycol, which can oxidize NO2- to NO3-. The specific calculation methods for each gas are described in the Supplementary Information S1 and S2.
The samples of fog water, bulk deposition, throughfall, stemflow, and gases mentioned above were collected every month, although the samples of wet deposition couldn’t be collected for freezing during January to March.
2.3 Analytical method
Samples of bulk deposition, throughfall, stemflow, and fog water were filtered through a 0.45 μm pore size membrane filter (A045A025A; Advantec, Co.) immediately after arrival in the laboratory. The electrical conductivity and pH were measured as soon as possible using electric conductivity (LAQUA DS-72; Horiba Ltd.) and pH meters (HM-42X; DKK-TOA Corp.), respectively. The concentrations of major inorganic ions (NH4+, Na+, K+, Mg2+, Ca2+, Cl−, NO3−, and SO42−) were determined using an ion chromatograph (Dionex Corp., DX-120 with a column from Dionex Corp., IonPac CS12A) for cations and an ion chromatograph (Dionex Corp., DX-120 with a column of Dionex Corp., IonPac AS12A) for anions. The concentrations of dissolved organic carbon (DOC) and dissolved inorganic carbon (DIC) were measured using a TOC analyzer (TOC-VCPH; Shimadzu Corp.) combined with an automatic sample injector (ASI-V; Shimadzu Corp.). Data with a cation:anion ratio greater than 1.5 or less than two thirds were excluded from the subsequent data analysis. The excluded data were 16.7% of the total data. However, even if the above conditions were not met, stemflow data were used because they contain unidentified organic acids that are difficult to analyze (Sato & Takahashi, 1996).
Each filter used to collect gaseous components, such as NH3, HNO3, HCl, SO2, and O3, was placed in a 200 mL Teflon beaker, and ultrapure water (10 mL for acid and ammonia gases and 15 mL for ozone sampling) was added. Then, the beaker was sealed with parafilm (PM996; Bemis Mfg. Co.) to prevent contamination by ambient pollutants. The sample in the Teflon beaker was irradiated with an ultrasonic wave for 20 min to dissolve the water soluble components. Subsequently, it was filtered using a 0.45 μm pore size membrane filter (A045A025A; Advantec Toyo Kaisha Ltd.). The filtrate was analyzed for NH4+, Cl−, NO2−, NO3−, and SO42− using ion chromatography, as mentioned above.
2.4 Collecting data on air pollution in East Asia
To show the air pollution indices, we used the SO2 concentration data provided by the Beijing Municipal Ecology Environment Bureau (http://sthjj.Beijing.gov.cn) for Beijing, the Shanghai Municipal Bureau of Ecology and Environment (http://sthj.sh.gov.cn) for Shanghai; Ministry of Ecology and Environment of the Republic of China (http://mee.gov.cn) for Henan and Liaoning prefectures, Seoul Air Environment Information (http://cleanair.Seoul.or.kr) for Seoul, and the Japanese Ministry of the Environment (http://soramame.taikei.go.jp) for the Japanese region. Air pollution data for total concentration of NO and NO2 (NOx), oxidant (Ox), SO2, and suspended particle matter (SPM) at Isehara were provided by National Institute for Environmental Studies (https://www.nies.go.jp/igreen/td_disp.html).
2.5 Backward trajectory analysis
The 3 d backward trajectories of air parcels reaching the summit of Mt. Oyama were calculated using the NOAA HYSPLT 4 model. A meteorological dataset was used for the GDAS, and model vertical velocity data were used for vertical motion. The initial elevation reading of the trajectory analysis was 1252 m.
3 Results and Discussion
3.1 Long term change of air pollution at the base of Mt. Oyama
In Japan, air pollution was most severe in the 1960s, and the highly polluted state persisted until the 1970s (Kawamura, 1985; Uno et al., 1996) causing acid rain (Tamaki, 1988) and photochemical smog (Kawamura, 1972; Wakamatsu et al., 1981). Sulfur dioxide concentrations decreased to below 5 ppb after the 1990s, but NO2 concentrations fluctuated around 15 ppb from the 1980s to the 2000s. Although air quality improved in the 2000s, oxidant concentrations have continued to increase from the 1980s to the present (Akimoto, 2003; Yoshikado, 2004).
Figure 2 shows the long term changes in air pollutant concentrations (NOx, SPM, Ox, and SO2) from 1994 to 2019 in Isehara, which is located at the foot of Mt. Oyama. The NOx concentrations gradually decreased from 55 ppb in 1994 to 45 ppb in 2005. The value declined further from 2005 to 2010, reaching 15 ppb by 2019. Suspended particulate matter (SPM) of less than 10 μm gradually decreased from 40 μg m3 in 1995 to 16 μg m3 in 2019. In the Tokyo metropolitan area, the "Automobile NOx Law" was enacted in 1992 to address NOx pollution problems caused by existing vehicles in heavily populated urban areas. This regulation was amended in 2001 to strengthen existing NOx regulations and add provisions for PM regulations, this came into effect in 2002 as the "Automobile NOx and PM Law". The decrease in NOx emission can be attributed to this regulation. In contrast, the oxidant concentration gradually increased from 29 ppb in 1994 to 35 ppb in 2019.
Long term changes in the concentration of NOx, SPM, O3, and SO2 at Isehara, the base of Mt. Oyama, from 1994 to 2019 (The data from the National Institute for Environmental Studies (https://www.nies.go.jp/igreen/td_disp.html). The measurement of SO2 at Isehara was stopped in 2013
The SO2 concentrations decreased gradually from 6 ppb in 1994 but rose in 2000 and decreased to the same level in 2004, as in 1999. In August 2000, a large-scale eruption occurred at the valley on Miyakejima Island (139°31'34 "E, 34°05'27 "N, 775 m a.s.l.), 150 km south of Mt. Oyama in the Tanzawa Mountains. In December 2000, the average monthly SO2 emission rate peaked at 54 kt day-1, exponentially decreased to 7 kt day-1 by the end of 2002, and then remained constant until December 2003 (Kazahaya et al., 2004). The cumulative SO2 emission from the eruption from December 2000 to August 2003 was approximately 18 Mt, which is comparable to the emission of a large explosive eruption, such as Pinatubo in 1991. This is comparable to the anthropogenic SO2 emissions from China (20.4 Mt) in 2000 and approximately 20 times higher than the annual anthropogenic SO2 emissions from Japan (0.801 Mt) in 2000. Since 2001, SO2 emissions have exhibited a downward trend, with daily emissions falling below 400 t day-1 since 2013, and below ten of t day-1 since 2017 (Supplementary Information S3).
The abundance of NOx and volatile organic compounds (VOCs), the precursors of Ox, in urban cities allows to produce large amounts of Ox. Although the enactment of the "Automobile NOx Law" has led to a reduction in precursor emissions, the concentration of Ox is increasing, probably due to large amounts of precursor emissions from ships on the ocean (Cooper, 2001, 2003; Corbett, 2002), as well as transboundary air pollution from Ox generated in large foreign cities across the ocean (Akimoto et al., 1996; Hatakeyama et al., 2004).
3.2 Elevational variation of gaseous air pollutants in Mt. Oyama
Figure 3 shows elevational variations in the concentrations of NH3, acidic gases (HCl, HNO3, and SO2), and O3 in Mt. Oyama from 1994 to 1998 (hereinafter referred to as the 1990s) and 2015 to 2019 (hereinafter referred to as the 2010s). In the 1990s and 2010s, NH3 concentrations decreased with altitude, suggesting the influence of agricultural and urban emissions (Sakurai & Fujita, 2002; Yamamoto et al., 1995) at lower altitudes in Mt. Oyama. For acid gases, no elevation dependence was observed in both the 1990s and 2010s, but SO2 increased with elevation in the 2010s. The emission of domestic primary pollution sources has been controlled, and the concentration of SO2 has shown a decreasing trend at all altitudes in 2010 compared to the 1990s. However, the summit has remained at roughly the same level, because the summit is affected not only by domestic pollution but by transboundary pollution, see “Frequency and pollution sources of acid fog in Mt. Oyama” section for a detailed explanation. The SO2 concentration shown in Fig. 3 was much lower than that shown in Fig. 2. The SO2 data was obtained by the solution electrical conductivity method, which always has positive deviation and has a large relative error in a low concentration (Yoshinari, 2015). The SO2 concentration measured by the ultraviolet fluorescence method at Hadano, 9 km far from Isehara, was reported to be the level of 1ppb in 2010s (https://www.nies.go.jp/igreen/td_disp.html).
O3 concentration increased with the altitude in Mt. Oyama as reported earlier (Hatakeyama et al., 2004; Nguyen et al., 2017). O3 concentration distribution is influenced not only by meteorological factors (e.g., temperature, wind speed, and pressure), but also by processes in which the photochemical reaction of ozone production from the emitted NOx and VOCs (Atkinson, 2000). The photochemical reaction proceeds during transportation from urban areas, and high elevation sites get more intense solar radiation that can accelerate photochemical reactions to produce more ozone from precursor elements (Moore & Semple, 2009; Semple et al., 2016). Therefore, ozone concentration becomes higher with altitude.
The mean concentrations of NH3, HCl, HNO3, and SO2 in the air at all elevations of Mt. Oyama were 2.08 ± 0.33, 0.42 ± 0.10, 0.30 ± 0.01, and 0.87 ± 0.05 ppb, respectively, during the 1990s. The mean concentrations of NH3, HCl, HNO3, SO2, and O3 in the air were 0.77 ± 0.13, 0.29 ± 0.03, 0.27 ± 0.03, 0.52 ± 0.08, and 18.54 ± 3.33 ppb, respectively, during the 2010s. Both NH3 and acid gas concentrations showed a decreasing trend, with the highest reduction observed in NH3 (–63.01%) followed by SO2 (–39.73%).
3.3 Long term trend of bulk deposition and fog water in Mt. Oyama
Figure 4 represents the volume weighted mean (VWM) pH, total ion concentration (TIC), and its composition of bulk deposition and precipitation at 400, 940, and 1252 m a.s.l. on Mt. Oyama from 1994 to 2019. There was no clear interannual change in precipitation at any altitude, and the TIC at each altitude also showed no clear trend. However, the VWM pH increased from 1994 to 1999, decreased sharply in 2002, and then remained low at each altitude, especially at 940 m, until at least 2013. The sudden decrease in pH after 2001 was probably due to the eruption of the Miyakajima volcano. The increase in pH after the eruption was 1.34%, 2.66%, and 1.92% per y at 400, 940, and 1252 m a.s.l., respectively. Moreover, the acid dominance factor of bulk deposition was compared to the mean ratio of N / S, that is the concentration ratio of NO3- to non-sea-salt sulfate (nss-SO42-), over the study period, and it was 0.58, 0.56., and 0.53 at 400, 940, and 1252 m a.s.l, respectively. As shown in Fig. 3, the concentration of SO2 increased gradually with elevation, whereas the concentration of HNO3 did not significantly change with elevation. Therefore, the N/S ratio in the bulk deposition gradually decreased with increasing altitude. This indicates that sulfuric acid has a greater acidifying effect on bulk deposition than nitric acid at high altitudes on Mt. Oyama, especially in recent periods.
Figure 5 shows the collected sample volume, VWM pH, total ion concentration (TIC), and composition of fog water at 680, 940, 1062, and 1252 m a.s.l. on Mt. Oyama from 1994 to 2019. Fog water has a lower liquid water content, smaller particle size, and longer residence time than rainwater (Seinfeld, 1986). Moreover, the ion concentration of fog water is 5–20 times higher than that of rainwater (Beiderwieden et al., 2007) and is highly acidic. In this study, a sample of fog water was collected using a PFC. The sample collected by the PFC contained not only fog, but also drizzle and rain (Wang et al., 2021). Similar to bulk deposition, VWM fog water pH increased from 1994 to 1999, but decreased at all altitudes in some years after 2000 due to the Miyakejima eruption. The collected sample volume at 680 m a.s.l. was much smaller than that at other elevation sites; however, the dry deposition or evaporation of deposited fog water on the PFC cannot be ignored at 680 m a.s.l. The TIC decreased with increasing altitude because the pollutant concentration in the fog water was diluted by the freshly formed fog droplets during the movement of air mass in the case of upslope fog (Igawa et al., 1998).
Thus, with the improvement in air quality, VWM pH of bulk deposition and fog water increased significantly at all altitudes, with an overall increase of 0.70% and 2.58% per y for bulk deposition and fog water, respectively, at Mt. Oyama. Additionally, we observed the fastest increase in bulk deposition and fogwater at 400 m a.s.l. (+1.01% per y) and 940 m a.s.l. (+4.4% per y), respectively.
3.4 Elevational variation of bulk and net throughfall deposition fluxes of NO3 - and nss–SO4 2- in Mt. Oyama
Figure 6 shows the elevational changes in the bulk and net throughfall deposition of NO3- and nss–SO42- on Mt. Oyama from 1994 to 2019. The long-term changes in the throughfall are shown in Supplementary Information S4. The VWM pH values of the bulk deposition and fog water are also shown in Fig. 6. The difference in VWM pH between bulk deposition and fog water increased with altitude because the dependence on altitude differed between bulk deposition and fog water, as shown in Figs. 4 and 5.
Bulk deposition fluxes of NO3- and nss–SO42- on average was 54.8 (range:27.4 to 108.4 meq m-2 y-1) and 53.6 meq m-2 y-1 (range:12.1 to 127.6 meq m-2 y-1), respectively. Maruyama et al. (2010) reported bulk deposition fluxes of NO3- and nss–SO42- of 38.0 and 65.1 meq m-2 y-1, respectively, for the 3 y period of 2006–2008 at the southeast foot of Mt. Fuji (1300 m a.s.l.), with an average precipitation of 3477 mm y-1. The deposition fluxes of NO3- and nss–SO42- at the foot of Mt. Fuji were 1.33 and 1.70 times higher than those at the summit of Mt. Oyama (1252 m a.s.l.), respectively. For the 1994–2019 period at the summit of Mt. Oyama, the average precipitation was 1843 mm y-1, whereas the precipitation at the southeast foot of Mt. Fuji was approximately twice as high, suggesting that the higher deposition at the southeast foot of Mt. Fuji mainly reflects the differences in precipitation. Comparing the bulk deposition fluxes in other mountainous areas in Japan (Seto et al., 2007), the average deposition fluxes from 2000 to 2002 were 20.7 and 50.1 meq m-2 y-1 for NO3- and nss–SO42-, respectively, at Happo (1850 m a.s.l). At Yusuhara (870 m a.s.l), NO3- and nss–SO42- deposition were 17.1 and 44.2 meq m-2 y-1, respectively. At the summit of Mt. Oyama, NO3- deposition fluxes were 1.37 and 1.67 times higher than those at Happo and Yusuhara, respectively. This may be because Mt Oyama is close to the Tokyo metropolitan area and is susceptible to air pollution, whereas Happo and Yusuhara are far from urban areas. In contrast, nss–SO42- deposition at Happo and Yusuhara were 1.31 and 1.15 times higher than that at Mt. Oyama. Although the two sites are remote, the two sites are closer to the mainland than Mt. Oyama. Therefore, the impact of transboundary pollution was stronger and nss–SO42- deposition was higher than that of Mt. Oyama.
The net throughfall deposition (NTD) flux is estimated by subtracting the bulk deposition flux from the throughfall deposition flux. NTD consists of dry and fog deposition, although small amounts of atmospheric deposition components, such as rainwater, dry deposition, including gases and aerosols, and fog deposition are absorbed or exchanged into the canopy, and canopy leaching occurs (Balestrini & Tagliaferri, 2001; Lovett & Lindberg, 1984; Lu et al., 2020; Zhang et al., 2007). NTDs of Japanese cedar at 680 m a.s.l. were 143.7 and 101.7 meq m-2 year-1 for NO3- and nss–SO42-, at 940 m a.s.l. were 344.6 and 139.6 meq m-2 year-1 for NO3- and nss–SO42-, at 1252 m a.s.l. were 427.0 and 275.5 meq m-2 year-1 for NO3- and nss–SO42-, respectively.
There was little change in the bulk deposition fluxes of NO3-and nss–SO42-with altitude, while their NTD increased with altitude. Wang et al. (2021) reported that the frequency of fog at the summit of Mt. Oyama (1252 m a.s.l.) was 32%, but 13% at 680 m a.s.l. This increase in the NTD with altitude is contributed by fog deposition onto the forest canopy because there was little change in acidic gases with altitude, as shown in Fig. 3.
3.5 Long term changes in stemflow of cedar and fir at Mt. Oyama
Beneath the forest canopy, wet deposition falls not only via throughfall, but also stemflow on the forest floor. Figure 7 shows the long term changes in the composition, pH, TIC, and dissolved organic carbon (DOC) in the stemflows of cedar and fir at 890 m a.s.l. on Mt. Oyama from 1996 to 2019. Similar to throughfall, stemflow is affected by dry deposition and leaching from the tree itself, as well as by wet deposition on the canopy. Furthermore, stemflow has a higher concentration than throughfall because it has a longer contact time with the leaves, branches, and stems. Therefore, stemflow not only has high concentrations of inorganic ions, but also dissolved organic carbon. The pH value of stemflow was lower than that of other samples, and the pH of cedar trees was much lower than that of fir trees. The stemflow pH value were 3.7 and 4.6 for cedar and fir, respectively, and were similar to the reported values of 2.87–3.46 for cedar (Sato, 1996) and 4.2–4.8 for fir (Sassa, 1991). The average DOC were 43.5 mg L-1 and 55.5 mg L-1 for cedar and fir, respectively, and were both within the ranges reported for stemflow, 6–322 mg L-1 (Stubbins et al., 2017; Van Stan & Stubbins, 2018). The pH values showed an increasing trend in recent years with the improvement of the atmospheric environment and reduction of acid deposition (+1.17% y-1 and +0.82% y-1 for cedar and fir, respectively), although they were generally low. To investigate the acid components, the correlation between DOC and the difference between the total anion and cation concentrations was examined for the stemflow samples. This difference may correspond to the concentration of unanalyzed organic acid components. The sample numbers of cedar (Japanese cedar) and fir (Abies firma) were 556 and 360, respectively, and DOC was highly correlated with the difference (r = 0.25 for cedar; and r = 0.42 for fir). It can be inferred from the slope that the molar ratio of acidic functional groups to carbon atoms in stemflow was 3:20 for cedar and 1:10 for fir. The acid concentrations in the stemflow samples were estimated using the Gran plot method (Henriksen & Seip, 1980; Krupa et al., 1976; Molværsmyr & Lund, 1983). The stemflow of cedar and fir trees were found to contain approximately 4 meq L-1 and 1 meq L-1 organic acids, respectively. Organic acids were not detected using this plot method for throughfall, bulk deposition, or fog water. Identifying organic acids in stemflow may be a very important subject to tackle in the future.
3.6 Comparison of bulk deposition, throughfall, fog water composition, and the long term changes in their composition at the summit of Mt. Oyama
Figure 8a shows the total ion concentration, volume weighted mean pH, and composition of bulk deposition, fog water, and throughfall under cedar trees averaged for all data at the summit of Mt. Oyama during 1994–2019. Owing to the fact that throughfall components are affected by the cleaning effects of precipitation on the leaves of plants, rainwater, fog water, aerosols, and leaf components adhered to the leaf surface would flow down and be collected. Thus, the proportions of K+, Mg2+, and Ca2+ in the throughfall were greater than those in bulk deposition or fog water. Among these cations, the throughfall of calcium and potassium ions were particularly high. Potassium ions are typical components of plant leaching whereas calcium ions are a major component of the dry deposition. In addition, calcium ions in the leaves are readily exchanged with the acid components of deposition on the canopy (Shigihara et al., 2009).
Comparison of volume weighted mean pH, total ion concentration (TIC), composition of bulk deposition, fog water, and throughfall under cedar trees from 1994 to 2019 at the summit of Mt.Oyama (a) and Five year mean of anion composition in bulk deposition, fog water, and throughfall under cedar trees at the summit of Mt. Oyama over three periods: before Miyakejima volcano eruption (BMVE, 1995-1999), after Miyakejima volcano eruption (AMVE, 2002-2004), and recent days (2015-2019). Due to the missing data in 2000 and 2001, the 3 y average used for AMVE was from 2002 to 2004 (b)
Figure 8b represents the 5 y mean of anion composition (chloride, nitrate, and sulfate ions) in bulk deposition, fog water, and throughfall under cedar trees at the summit of Mt. Oyama classified for three periods, before the Miyakejima volcano eruption (BMVE, 1995-1999), after the Miyakejima volcano eruption (AMVE, 2002-2004), and recent days (2015-2019). Owing to missing data for 2000 and 2001, the 3 y average used for AMVE was from 2002 to 2004. The recent ratios of NO3- for all samples showed a significant decreasing trend compared to BMVE, while the ratio of Cl- increased, with the largest increase in fog water (+71.6%), followed by throughfall (+38.5%). This was caused by the reduction of NOx in the atmosphere, as shown in Fig. 2. Comparing AMVE with BMVE, SO42- ions significantly increased owing to the effect of the Miyakejima volcano eruption, especially for bulk deposition. The different trends among these three sample sets may be caused by the fact that bulk precipitation components are affected by air pollution over a wide region, while fog water components are primarily affected by air pollution in local subsurface regions. Throughfall affected not only precipitation, but also fog water.
Figure 9 shows the long term changes in concentration of nonmetallic cations, hydrogen ions, and ammonium ions for bulk deposition, fog water, and throughfall under the cedar at the summit of Mt. Oyama. The total concentration of [H+] + [NH4+] decreased linearly annually for bulk deposition, throughfall, and fog water. The concentration of [H+] + [NH4+] decreased in all samples, with the largest decrease in fog water (–0.37% y-1) and smallest in rainwater (–0.06% y-1). The major metallic cations, Na+, K+, Mg2+, and Ca2+, did not decrease in any of the samples. Figure 9b and c show the changes in concentration of the respective cations. It is evident that the rate of decrease in [NH4+] was greater than that of [H+] for all samples. The concentration of ammonium ions in wet deposition was dominated by not only the ambient ammonia gas concentration, but also the neutralization reaction with the acid in the deposition. Both the ammonia gas concentration and acidity of the wet deposition decreased on Mt. Oyama. The slope of [H+] of the throughfall was much smaller than that of [NH4+]. This can be explained by the ion exchange reaction of H+ with the leaf components in fog (Igawa, Kase, et al., 2002).
3.7 Frequency and pollution sources of acid fog in Mt. Oyama
Figure 10a represents the frequency of acid fog classified into four levels by its pH value at the summit of Mt. Oyama from 1994 to 2019 in summer and winter. Figure 10b shows the average values of fog water concentration and pH in the summer and winter from 1994 to 1997 and 2015 to 2019. From January to March, the temperature at the top of the mountain was too low, and the water samples were frozen. Thus, samples could not be collected. Hence, the samples from April and December were used as the winter samples in which transboundary pollution was highly affected (Inomata et al., 2016). In summer, it is apparent that the frequency of acidic fog with low pH gradually decreased, as shown in Fig. 10a. As shown in Fig. 10b, the summer fog water pH increased from 4.14 in 1994–1997 to 4.81 in 2015–2019 (+16.2%). In contrast, in winter, except for the duration of the eruption of the volcano on Miyakejima Island in 2000 and several small-scale eruptions of Mt. Mitakesan volcano in the Nagano prefecture in 2007, there were few marked changes. The pH value of fog water in summer increased, whereas the pH ratio in winter did not change, as shown in Fig. 10a. The changes in total ion concentration of fog water in winter (23.68%) was smaller than that in summer (45.45%). The pH of fog water in winter was 4.43 in 1994–1997 and 4.41 in 2015–2019. Thus, there was barely any change.
To infer the cause of this dependence of the fog water pH on the seasons, we used a backward trajectory to verify the air mass source (Fig. 11). Figure 11a represents the summer of 2019: most of the air masses reaching the summit of Mt. Oyama came from the Pacific Ocean through mainland Japan, although the air mass moved in various directions. The trajectories were approximately the same for each year. Therefore, in summer, the pollution source of Mt. Oyama was mainly mainland Japan. Land and sea breezes occur frequently in summer. Sea breezes discharge air pollutants from coastal cities and suburbs to inland areas (Bonsang et al., 2001). This cycle facilitates the accumulation of pollutants and generation of secondary pollutants under the influence of photochemistry (Kallos et al., 1993; Kotroni et al., 1999; Liu & Chan, 2002). In recent years, improvements in the atmospheric environment have reduced the number of primary pollutants and mitigated the generation of secondary pollutants (Fig. 2). As shown in Fig. 10a, the frequency of acid fog in summer decreased with a decrease in acidic pollutants. In winter, it is apparent that air masses from the Asian continent account for most of the pollutants (Fig. 11b). China uses coal for 70% of its total energy needs (Fang et al., 2009), which has become a major anthropogenic contributor to air pollution in China. This phenomenon is especially prominent during the winter due to the use of central heating (Chen et al., 2017; Shen et al., 2013; Wang et al., 2003). A large increase in coal combustion resulted in significant emissions. These pollutants, accompanied by the movement of air masses from northern China, northeastern China, and northwestern China, through Korea, reach western Japan (Takami et al., 2005, 2007), and further affect the ambient air around Mt. Oyama.
To prove our hypothesis, we compared the concentration changes of SO2 in different regions of China, Korea, and Japan in winter along a backward trajectory. Figure 12a shows the average concentration changes in SO2 in four prefectures of China (Beijing, Shanghai, Henan, Liaoning), one prefecture of South Korea (Seoul), three prefectures of Japan (Fukuoka, Hyogo, and Nagano), and the top of Mt. Oyama (1252 m a. s.l.) in the winter of 2018 (December 2018 to February 2019). During the monsoon season, the average concentration of SO2 decreased sequentially in the three countries. Pollutants flowed into Japan from China, and Mt. Oyama was also affected by transboundary pollution in winter. The above in Fig. 12b represents the backward trajectories every six hours (0:00, 6:00, 12:00, 18:00, and 24:00 JST) from Mt. Oyama on February 2, 2019, and the calculation time was 72 h (Meters AGL:1252m). Figure 12b shows the temporal variation in SO2 concentration detected at different measuring points in different countries when the air mass flows through them. We found, from the temporal variations, that an air mass containing high concentrations of SO2 flowed down from mainland China to Japan via South Korea. The bottom half of Fig. 12 shows that Mt. Oyama was affected by transboundary pollution, which lead to an increase in the frequency of acid fog in winter.
SO2 concentration at 4 prefectures in China (Beijing, Shanghai, Liaoning, Henan), 1 prefecture in Korea (Seoul), 3 prefectures (Fukuoka, Hyogo, Nagano) and the summit of Mt. Oyama in Japan in 2018 winter and the flow of monsoon in winter (a); temporal variation of backward trajectory and SO2 concentration at different cities (b)
4 Conclusions
The concentrations of acid pollutants tended to decrease with the improvement of the domestic atmospheric environment in Japan, and the pH of precipitation (including bulk deposition, fog water, and throughfall) increased in the quarter century until 2019. The bulk deposition flux of acids, NO3-, and nss-SO42- barely changed with altitude, whereas the NTD increased with increasing altitude, probably due to fog water deposition. The pH of stemflow was low because of the influence of organic acids dissolved from the tree itself, but also increased because of the reduction of air pollutants.
In summer, sea breezes transport air pollutants generated at coastal urban sites, and fog water is prone to be acid. However, the frequency of acid fog in summer has been decreasing with improvements in the domestic environment. Under the influence of monsoons in winter, high concentrations of pollutants from distant areas flowed into Japan, and the frequency of acid fog remained almost unchanged. Drastic damage on mountain forest will not occur in the future for the decreasing severe acid fog events, but further improvements in the domestic atmospheric environment and control of transboundary pollution are expected to provide better conditions for mountain ecology free from acid pollutants.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abe, M., Oniwa, S., Okochi, H., & Aikawa, M. (2022). Fog Water, Throughfall, and Bulk Precipitation Chemistry and a Newly Developed Methodology for the Estimation of Fog Water Deposition. Water, Air, & Soil Pollution, 233(11), 474. https://doi.org/10.1007/s11270-022-05939-5
Aikawa, M., Hiraki, T., & Tamaki, M. (2006). Comparative field study on precipitation, throughfall, stemflow, fog water, and atmospheric aerosol and gases at urban and rural sites in Japan. The Science of the Total Environment, 366(1), 275–285. https://doi.org/10.1016/j.scitotenv.2005.06.027
Akimoto, H. (2003). Recent photochemical smog issue in Japan. Journal of Environmental Conservation Engineering, 32(7), 510–516. https://doi.org/10.5956/jriet.32.510
Akimoto, H., Mukai, H., Nishikawa, M., Murano, K., Hatakeyama, S., Liu, C., Buhr, M., Hsu, K. J., Jaffe, D. A., Zhang, L., Honrath, R., Merrill, J. T., & Newell, R. E. (1996). Long-Range Transport of Ozone in the East Asian Pacific Rim Region. Journal of Geophysical Research, 101, 1999–2010. https://doi.org/10.1029/95JD00025
Al-Alawi, M., Batarseh, M. I., Carreras, H., Alawi, M., Jiries, A., & Charlesworth, S. M. (2007). Aleppo pine bark as a biomonitor of atmospheric pollution in the arid environment of Jordan. CLEAN-Soil Air Water, 35, 438–443. https://doi.org/10.1002/clen.200720017
Al-Alawi, M., & Mandiwana, K. L. (2007). The use of Aleppo pine needles as a bio-monitor of heavy metals in the atmosphere. Journal of Hazerdous Materials, 148, 43–46. https://doi.org/10.1016/j.jhazmat.2007.02.001
Anderson, J. B., Baumgardner, R. E., Mohnen, V. A., & Bowser, J. J. (1999). Cloud chemistry in the eastern United States, as sampled from three high-elevation sites along the Appalachian Mountains. Atmospheric Environment, 33(30), 5105–5114. https://doi.org/10.1016/S1352-2310(99)00193-4
Atkinson, R. (2000). Atmospheric chemistry of VOCs and NOx. Atmospheric Environment, 34(12), 2063–2101. https://doi.org/10.1016/S1352-2310(99)00460-4
Avila, A. (1996). Time trends in the precipitation chemistry at a mountain site in Northeastern Spain for the period 1983–1994. Atmospheric Environment, 30(9), 1363–1373. https://doi.org/10.1016/1352-2310(95)00472-6
Balestrini, R., & Tagliaferri, A. (2001). Atmospheric deposition and canopy exchange processes in alpine forest ecosystems (northern Italy). Atmospheric Environment, 35(36), 6421–6433. https://doi.org/10.1016/S1352-2310(01)00350-8
Batarseh, M., Ziadat, A., Al-Alawi, M., Berdanier, B., & Jiries, A. (2008). The use of cypress tree bark as an environmental indicator of heavy metals deposition in Fuheis City, Jordan. International Journal of Environment and Pollution, 33, 207. https://doi.org/10.1504/IJEP.2008.019394
Beiderwieden, E., Schmidt, A., Hsia, Y. J., Chang, S. C., Wrzesinsky, T., & Klemm, O. (2007). Nutrient Input Through Occult and Wet Deposition into a Subtropical Montane Cloud Forest. Water, Air, and Soil Pollution, 186(1), 273–288. https://doi.org/10.1007/s11270-007-9483-0
Bian, Y., & Yu, S. (1992). Forest decline in Nanshan, China. Forest Ecology and Management, 51(1), 53–59. https://doi.org/10.1016/0378-1127(92)90471-K
Bonsang, B., Moortgat, G. K., & Pio, C. A. (2001). Overview of the FIELDVOC’94 experiment in a eucalyptus forest of Portugal. Chemosphere - Global Change Science, 3(3), 211–226. https://doi.org/10.1016/S1465-9972(01)00006-X
Bussotti, F., & Ferretti, M. (1998). Air pollution, forest condition and forest decline in Southern Europe: An overview. Environmental Pollution, 101(1), 49–65. https://doi.org/10.1016/S0269-7491(98)00039-6
Chen, S., Xu, L., Zhang, Y., Chen, B., Wang, X., Zhang, X., Zheng, M., Chen, J., Wang, W., Sun, Y., Fu, P., Wang, Z., & Li, W. (2017). Direct observations of organic aerosols in common wintertime hazes in North China: insights into direct emissions from Chinese residential stoves. Atmospheric Chemistry and Physics, 17(2), 1259–1270. https://doi.org/10.5194/acp-17-1259-2017
Chiwa, M., Kim, D. H., & Sakugawa, H. (2003). Rainfall, Stemflow, and Throughfall Chemistry at Urban- and Mountain-facing Sites at Mt. Gokurakuji, Hiroshima, Western Japan. Water, Air, and Soil Pollution, 146(1), 93–109. https://doi.org/10.1023/A:1023946603217
Cooper, D. A. (2001). Exhaust emissions from high speed passenger ferries. Atmospheric Environment, 35, 4189–4200. https://doi.org/10.1016/S1352-2310(01)00192-3
Cooper, D. A. (2003). Exhaust emissions from ships at berth. Atmospheric Environment, 37, 3817–3830. https://doi.org/10.1016/S1352-2310(03)00446-1
Corbett, J. J. (2002). Emissions from ships in the northwestern United States. Environmental Science & Technology, 36(6), 1299–1306. https://doi.org/10.1021/es0155985
Dai, Z., Liu, Y., Wang, X., & Zhao, D. (1998). Changes in pH, CEC and Exchangeable Acidity of Some Forest Soils in Southern China During the Last 32–35 Years. Water, Air, and Soil Pollution, 108(3), 377–390. https://doi.org/10.1023/A:1005015526022
Fang, M., Chan, C. K., & Yao, X. (2009). Managing air quality in a rapidly developing nation: China. Atmospheric Environment, 43(1), 79–86. https://doi.org/10.1016/j.atmosenv.2008.09.064
Hatakeyama, S., Katahira, K., Takami, A., Sugata, S., Liu, F., & Kita, K. (2004). Variation of Concentration of Ozone in Summer and Autumn in Oku-Nikko mountainous area. Journal of Japan Society for Atmospheric Environment / Taiki Kankyo Gakkaishi, 39(3), 158–170. https://doi.org/10.11298/taiki1995.39.3_158
Hatakeyama, S., Takami, A., Sakamaki, F., Mukai, H., Sugimoto, N., Shimizu, A., & Bandow, H. (2004). Aerial measurement of air pollutants and aerosols during 20–22 March 2001 over the East China Sea. Journal of Geophysical Research: Atmospheres, 109(D13). https://doi.org/10.1029/2003JD004271
Henriksen, A., & Seip, H. M. (1980). Strong and weak acids in surface waters of southern Norway and southwestern Scotland. Water Research, 14(7), 809–813. https://doi.org/10.1016/0043-1354(80)90261-4
Horn, K. J., Thomas, R. Q., Clark, C. M., Pardo, L. H., Fenn, M. E., Lawrence, G. B., Perakis, S. S., Smithwick, E. A. H., Baldwin, D., Braun, S., Nordin, A., Perry, C. H., Phelan, J. N., Schaberg, P. G., Clair, S. B., Warby, R., & Watmough, S. (2018). Growth and survival relationships of 71 tree species with nitrogen and sulfur deposition across the conterminous US. PloS One, 13(10), e0205296. https://doi.org/10.1371/journal.pone.0205296
Igawa, M., Kase, T., Satake, K., & Okochi, H. (2002). Severe leaching of calcium ions from fir needles caused by acid fog. Environmental Pollution, 119(3), 375–382.
Igawa, M., Kojima, K., Yoshimoto, O., & Nanzai, B. (2015). Air pollutant deposition at declining forest sites of the Tanzawa Mountains, Japan. Atmospheric Research, 151, 93–100. https://doi.org/10.1016/j.atmosres.2014.03.017
Igawa, M., Matsumura, K., & Okochi, H. (2002). High Frequency and Large Deposition of Acid Fog on High Elevation Forest. Environmental Science & Technology, 36(1), 1–6. https://doi.org/10.1021/es0105358
Igawa, M., Nagaike, E., Nakayama, M., Matsumoto, K., & Uchiyama, Y. (2007). Concentrations of trace gaseous species in Tanzawa Mountains. In Results of the Scientific Research on the Tanzawa Mountains, Kanagawa Pref (pp. 403–404).
Igawa, M., Tsutsumi, Y., Mori, T., & Okochi, H. (1998). Fogwater Chemistry at a Mountainside Forest and the Estimation of the Air Pollutant Deposition via Fog Droplets Based on the Atmospheric Quality at the Mountain Base. Environmental Science & Technology, 32(11), 1566–1572. https://doi.org/10.1021/es970213x
Inomata, Y., Ohizumi, T., Take, N., Sato, K., & Nishikawa, M. (2016). Transboundary transport of anthropogenic sulfur in PM2.5 at a coastal site in the Sea of Japan as studied by sulfur isotopic ratio measurement. Science of The Total Environment, 553, 617–625. https://doi.org/10.1016/j.scitotenv.2016.02.139
Johnson, A. H., & Siccama, T. G. (1983). Acid deposition and forest decline. Environmental Science & Technology, 17(7), 294A–305A. https://doi.org/10.1021/es00113a717
Kallos, G., Kassomenos, P., & Pielke, R. A. (1993). Synoptic and mesoscale weather conditions during air pollution episodes in Athens. Greece. Boundary-Layer Meteorology, 62(1), 163–184. https://doi.org/10.1007/BF00705553
Kawamura, T. (1972). Air Pollution in Uruban Areas in Janpan. Geographical Review of Japan, 45(3), 231–245. https://doi.org/10.4157/grj.45.231
Kawamura, T. (1985). Recent Changes of Atmospheric Environment in Tokyo and Its Surrounding Area. Geographical Review of Japan, Series B., 58(1), 83–94. https://doi.org/10.4157/grj1984b.58.83
Kazahaya, K., Shinohara, H., Uto, K., Odai, M., Nakahori, Y., Mori, H., Hideki, L., Miyashita, M., & Hirabayashi, J. (2004). Gigantic SO2 emission from Miyakejima volcano, Japan, caused by caldera collapse. Geology, 32(5), 425–428. https://doi.org/10.1130/G20399.1
Kim, M. H., & Lee, S. W. (1993). Injury responses of woody landscape plants to air pollutants (I)-sulfur and heavy metal content. Journal of Korean Society of Forest Science, 82(3), 271–282.
Kiseleva, V. (1996). Environmental Stress to the Siberian forests: An overview (Vol. 34). IIASA Working Paper.
Kohno, Y. (2017). In T. Izuta (Ed.), Decline of Fagus crenata in the Tanzawa Mountains, Japan BT - Air Pollution Impacts on Plants in East Asia (pp. 151–162). Springer Japan. https://doi.org/10.1007/978-4-431-56438-6_10
Kohno, Y., Matsumura, H., Ishii, T., & Izuta, T. (2005). Establishing critical levels of air pollutants for protecting East Asian vegetation—a challenge. In Plant responses to air pollution and global change (pp. 243–250). Springer.
Kosiba, A. M., Schaberg, P. G., Rayback, S. A., & Hawley, G. J. (2018). The surprising recovery of red spruce growth shows links to decreased acid deposition and elevated temperature. Science of The Total Environment, 637–638, 1480–1491. https://doi.org/10.1016/j.scitotenv.2018.05.010
Kotroni, V., Kallos, G., Lagouvardos, K., Varinou, M., & Walko, R. (1999). Numerical Simulations of the Meteorological and Dispersion Conditions during an Air Pollution Episode over Athens. Greece. Journal of Applied Meteorology, 38(4), 432–447. https://doi.org/10.1175/1520-0450(1999)038<0432:NSOTMA>2.0.CO;2
Krupa, S., Coscio, M. R., & Wood, F. A. (1976). Evaluation of a Coulometric Procedure For the Detection of Strong and Weak Acid Components in Rainwater. Journal of the Air Pollution Control Association, 26(3), 221–223. https://doi.org/10.1080/00022470.1976.10470246
Lawrence, D. M., Fisher, R. A., Koven, C. D., Olsen, K. W., Swenson, S. C., Bonan, G., Collier, N., Ghimire, B., Kampenhout, L., Kennedy, D., Kluzek, E., Lawrence, P. J., Li, F., Li, H., Lombardozzi, D., Riley, W. J., Sacks, W. J., Shi, M., Vertenstein, M., et al. (2019). The Community Land Model version 5: Description of new features, benchmarking, and impact of forcing uncertainty. Journal of Advances in Modeling Earth Systems, 11(12), 4245–4287. https://doi.org/10.1029/2018MS001583
Likens, G. E., Driscoll, C. T., & Buso, D. C. (1996). Long-Term Effects of Acid Rain: Response and Recovery of a Forest Ecosystem. Science, 272, 244–246. https://doi.org/10.1126/science.272.5259.244
Liu, H., & Chan, J. C. L. (2002). An investigation of air-pollutant patterns under sea–land breezes during a severe air-pollution episode in Hong Kong. Atmospheric Environment, 36(4), 591–601. https://doi.org/10.1016/S1352-2310(01)00504-0
Lovett, G. M., & Lindberg, S. E. (1984). Dry deposition and canopy exchange in a mixed oak forest as determined by analysis of throughfall. Journal of Applied Ecology, 1013–1027. https://doi.org/10.2307/2405064
Lu, X., Liu, Y., Liu, L., Zhang, Z., Hu, F., Liu, X., & Sun, X. (2020). Rainfall partitioning and associated nitrate and sulfate fluxes along a slope gradient in a subtropical broadleaved forest. Journal of Hydrology, 591, 125584. https://doi.org/10.1016/J.JHYDROL.2020.125584
Maes, S. L., Perring, M. P., Vanhellemont, M., Depauw, L., Van den Bulcke, J., Brūmelis, G., Brunet, J., Decocq, G., Ouden, J., Härdtle, W., Hédl, R., Heinken, T., Heinrichs, S., Jaroszewicz, B., Kopecký, M., Máliš, F., Wulf, M., & Verheyen, K. (2019). Environmental drivers interactively affect individual tree growth across temperate European forests. Global Change Biology, 25(1), 201–217. https://doi.org/10.1111/gcb.14493
Maruyama, S., Okochi, H., Takemura, N., & Minami, Y. (2010). Long-Term Performance of a Solar-Powered Small Automatic Rainwater Collector and Its Application to Acid Deposition Monitoring in the Southeast Mountainside of Mt. Fuji. Bunseki Kagaku, 59(5), 357–362. https://doi.org/10.2116/bunsekikagaku.59.357
Menz, F. C., & Seip, H. M. (2004). Acid rain in Europe and the United States: an update. Environmental Science & Policy, 7(4), 253–265. https://doi.org/10.1016/j.envsci.2004.05.005
Millar, C. I., & Stephenson, N. L. (2015). Temperate forest health in an era of emerging megadisturbance. Science, 349(6250), 823–826.
Molværsmyr, K., & Lund, W. (1983). Acids and bases in fresh-waters: Interpretation of results from gran plots. Water Research, 17(3), 303–307. https://doi.org/10.1016/0043-1354(83)90184-7
Moore, G. W. K., & Semple, J. L. (2009). High concentration of surface ozone observed along the Khumbu Valley Nepal April 2007. Geophysical Research Letters, 36(14). https://doi.org/10.1029/2009GL038158
Nguyen, H. T., Kwon, E. E., Kim, K.-H., Pandey, S. K., Chambers, S., Kumar, P., Kang, C.-H., Cho, S.-J., Oh, J.-M., & Brown, R. J. C. (2017). Factors regulating the distribution of O3 and NOx at two mountainous sites in Seoul, Korea. Atmospheric Pollution Research, 8(2), 328–337. https://doi.org/10.1016/j.apr.2016.10.003
Nowak, D. J., Crane, D. E., Stevens, J. C., Hoehn, R. E., Walton, J. T., & Bond, J. (2008). A ground-based method of assessing urban forest structure and ecosystem services. Aboriculture & Urban Forestry, 34(6), 347–358.
Ohse, K., Ohkawa, Y., Tamura, K., & Higashi, T. (2003). Influences of forest decline on various properties of soils on Mt. Hirugatake, Tanzawa Mountains, Kanto District, Japan. Soil Science and Plant Nutrition, 49(2), 171–177. https://doi.org/10.1080/00380768.2003.10409994
Pannatier, E. G., Thimonier, A., Schmitt, M., Walthert, L., & Waldner, P. (2011). A decade of monitoring at Swiss Long-Term Forest Ecosystem Research (LWF) sites: can we observe trends in atmospheric acid deposition and in soil solution acidity? Environmental Monitoring and Assessment, 174(1–4), 3–30. https://doi.org/10.1007/s10661-010-1754-3
Paoletti, E., Schaub, M., Matyssek, R., Wieser, G., Augustaitis, A., Bastrup-Birk, A. M., Bytnerowicz, A., Günthardt-Goerg, M. S., Müller-Starck, G., & Serengil, Y. (2010). Advances of air pollution science: From forest decline to multiple-stress effects on forest ecosystem services. Environmental Pollution, 158(6), 1986–1989. https://doi.org/10.1016/j.envpol.2009.11.023
Phu Nguyen, L. S., Zhang, L., Lin, D.-W., Lin, N.-H., & Sheu, G.-R. (2019). Eight-year dry deposition of atmospheric mercury to a tropical high mountain background site downwind of the East Asian continent. Environmental Pollution, 255, 113128. https://doi.org/10.1016/j.envpol.2019.113128
Pitelka, L. F., & Raynal, D. J. (1989). Forest Decline and Acidic Deposition. Ecology, 70(1), 2–10. https://doi.org/10.2307/1938405
Ren, W., Tian, H., Tao, B., Chappelka, A., Sun, G., Lu, C., Liu, M., Chen, G., & Xu, X. (2011). Impacts of tropospheric ozone and climate change on net primary productivity and net carbon exchange of China’s forest ecosystems. Global Ecology and Biogeography, 20(3), 391–406. https://doi.org/10.1111/J.1466-8238.2010.00606.X
Sakurai, T., & Fujita, S. (2002). Analysis of atmospheric ammonia budget for the Kanto region, Japan. Atmospheric Environment, 36(26), 4201–4209. https://doi.org/10.1016/S1352-2310(02)00349-7
Sassa, T. (1991). Acidity and nutrient elements of the rain fall, throughfall and stem flow in the typical forests around Morioka City, Iwate Pref., Japan. Japanese Journal of Forest Environment, 32, 43–58.
Sassa, T., Goto, K., Hasegawa, K., & Ikeda, S. (1990). Acidity and Nutrient Elements of the Rain fall, Through fall and Stem flow in the Typical forests around Morioka City, Iwate Pref., Japan: Characteristic pH value of the Stem flow in several Tree species. Japanese Journal of Forest Environment, 32(2), 43–58. https://doi.org/10.18922/jjfe.32.2_43
Wang, Y., Okochi, H., & Igawa, M. (2021). Characteristics of fog and fog collection with passive collector at Mt. Oyama in Japan. Water, Air, & Soil Pollution, 232(7), 1–15. https://doi.org/10.1007/S11270-021-05205-0
Sato, K. (1996). Leaching of H+ from stem of Japanese red cedar and soil acidification around the stem. Environmental Science, 9, 221–230.
Sato, K., & Takahashi, A. (1996). Hydrogen Ion Leaching from the Stem of a Japanese Cedar Tree and Acidification of Soils around the Stem. Environment Science, 9(2), 221–230. https://doi.org/10.11353/sesj1988.9.221
Schutt, P., & Cowling, E. B. (1985). Waldsterben, a general decline of forests in central Europe: symptoms, development, and possible causes. Plant Disease, 69(7), 548–558.
Seinfeld, J. H. (1986). ES&T books: atmospheric chemistry and physics of air pollution. Environmental Science & Technology, 20(9), 863. https://doi.org/10.1021/es00151a602
Sekiguchi, K., Hara, Y., & Ujiiye, A. (1986). Dieback of cryptomeria Japonica and distribution of acid deposition and oxidant in Kanto district of Japan. Environmental Technology Letters, 7(1–12), 263–268. https://doi.org/10.1080/09593338609384411
Semple, J. L., Moore, G. W. K., Koutrakis, P., Wolfson, J. M., Cristofanelli, P., & Bonasoni, P. (2016). High Concentrations of Ozone Air Pollution on Mount Everest: Health Implications for Sherpa Communities and Mountaineers. High Altitude Medicine & Biology, 17(4), 365–369. https://doi.org/10.1089/ham.2016.0042
Seto, S., Sato, M., Tatano, T., Kusakari, T., & Hara, H. (2007). Spatial distribution and source identification of wet deposition at remote EANET sites in Japan. Atmospheric Environment, 41(40), 9386–9396. https://doi.org/10.1016/J.ATMOSENV.2007.09.002
Shen, G., Wei, S., Zhang, Y., Wang, B., Wang, R., Shen, H., Li, W., Huang, Y., Chen, Y., Chen, H., & Tao, S. (2013). Emission and size distribution of particle-bound polycyclic aromatic hydrocarbons from residential wood combustion in rural China. Biomass and Bioenergy, 55, 141–147. https://doi.org/10.1016/j.biombioe.2013.01.031
Shigihara, A., Matsumoto, K., Sakurai, N., & Igawa, M. (2008). Growth and physiological responses of beech seedlings to long-term exposure of acid fog. Science of the Total Environment, 391(1), 124–131. https://doi.org/10.1016/j.scitotenv.2007.10.053
Shigihara, A., Matsumura, Y., Matsumoto, K., & Igawa, M. (2009). Effect of simulated acid fog on membrane-bound calcium (mCa) in fir (Abies firma) and cedar (Cryptomeria japonica) mesophyll cells. Journal of Forest Research, 14, 188–192. https://doi.org/10.1007/s10310-009-0120-1
Stubbins, A., Silva, L. M., Dittmar, T., & Van Stan, J. T. (2017). Molecular and Optical Properties of Tree-Derived Dissolved Organic Matter in Throughfall and Stemflow from Live Oaks and Eastern Red Cedar. Frontiers in Earth Science, 5. https://doi.org/10.3389/feart.2017.00022
Suzuki, K. (1992). Fluctuation of Abies firma dead standing trees and change of annual ring width at Mt. Oyama and the around areas in Kanagawa Prefecture. Bulletin of the Kanagawa Prefecture Forest Experiment Station, 19, 23–42.
Tago, H., Kimura, H., Kozawa, K., & Fujie, K. (2006). Long-Term Observation of Fogwater Composition at two Mountainous Sites in Gunma Prefecture, Japan. Water, Air, and Soil Pollution, 175(1), 375–391. https://doi.org/10.1007/s11270-006-9144-8
Takahashi, M., Feng, Z., Mikhailova, T. A., Kalugina, O. V., Shergina, O. V., Afanasieva, L. V., Heng, R. K. J., Majid, N. M. N. A., & Sase, H. (2020). Air pollution monitoring and tree and forest decline in East Asia: A review. Science of The Total Environment, 742, 140288. https://doi.org/10.1016/J.SCITOTENV.2020.140288
Takami, A., Miyoshi, T., Shimono, A., & Hatakeyama, S. (2005). Chemical composition of fine aerosol measured by AMS at Fukue Island, Japan during APEX period. Atmospheric Environment, 39(27), 4913–4924. https://doi.org/10.1016/j.atmosenv.2005.04.038
Takami, A., Miyoshi, T., Shimono, A., Kaneyasu, N., Kato, S., Kajii, Y., & Hatakeyama, S. (2007). Transport of anthropogenic aerosols from Asia and subsequent chemical transformation. Journal of Geophysical Research: Atmospheres, 112(D22). https://doi.org/10.1029/2006JD008120
Takeda, M., & Aihara, K. (2007). Effects of Ambient Ozone Concentrations on Beech (Fagus crenata) Seedlings in the Tanzawa Mountains, Kanagawa Prefecture, Japan. Journal of Japan Society for Atmospheric Environment / Taiki Kankyo Gakkaishi, 42(2), 107–117. https://doi.org/10.11298/taiki1995.42.2_107
Takeuchi, M., Okochi, H., & Igawa, M. (2004). Characteristics of water-soluble components of atmospheric aerosols in Yokohama and Mt. Oyama, Japan from 1990 to 2001. Atmospheric Environment, 38(28), 4701–4708. https://doi.org/10.1016/j.atmosenv.2004.05.027
Tamaki, M. (1988). Acid Deposition in Japan. Journal of Environmental Conservation Engineering, 17(11), 696–704. https://doi.org/10.5956/jriet.17.696
Tanimoto, H. (2009). Increase in springtime tropospheric ozone at a mountainous site in Japan for the period 1998–2006. Atmospheric Environment, 43(6), 1358–1363. https://doi.org/10.1016/j.atmosenv.2008.12.006
Uno, I., Ohara, T., & Wakamatsu, S. (1996). Analysis of wintertime NO2 pollution in the Tokyo metropolitan area. Atmospheric Environment, 30(5), 703–713. https://doi.org/10.1016/1352-2310(95)00177-8
Van Stan, J. T., & Stubbins, A. (2018). Tree-DOM: Dissolved organic matter in throughfall and stemflow. Limnology and Oceanography Letters, 3(3), 199–214. https://doi.org/10.1002/lol2.10059
Vong, R. J., Sigmon, J. T., & Mueller, S. F. (1991). Cloud water deposition to Appalachian forests. Environmental Science & Technology, 25(6), 1014–1021. https://doi.org/10.1021/es00018a002
Wakamatsu, S., Ogawa, Y., Murano, K., Okuda, M., Tsuruta, H., Goi, K., & Aburamoto, Y. (1981). Aircraft Survey of Photochemical Smog in Tokyo Metropolitan Area. Journal of Japan Society of Air Pollution, 16(4), 199–214. https://doi.org/10.11298/taiki1978.16.199
Yoshinari, H. (2015). The history examined sulfur dioxide measurement methods. Journal of Japan Society for Atmospheric Environment, 50(3), 29–37. https://doi.org/10.11298/taiki.50.A29
Wang, G., Wang, H., Yu, Y., Gao, S., Feng, J., Gao, S., & Wang, L. (2003). Chemical characterization of water-soluble components of PM10 and PM2.5 atmospheric aerosols in five locations of Nanjing, China. Atmospheric Environment, 37(21), 2893–2902. https://doi.org/10.1016/S1352-2310(03)00271-1
Watanabe, K., Ji, J., Harada, H., Sunada, Y., & Honoki, H. (2022). Recent Characteristics of Fog Water Chemistry at Mt. Tateyama, Central Japan: Recovery from High Sulfate and Acidity. Water, Air, & Soil Pollution, 233(8), 300. https://doi.org/10.1007/s11270-022-05778-4
Watmough, S. A., Eimers, C., & Baker, S. (2016). Impediments to recovery from acid deposition. Atmospheric Environment, 146, 15–27. https://doi.org/10.1016/j.atmosenv.2016.03.021
Wright, A. L., & Hons, F. M. (2005). Soil Carbon and Nitrogen Storage in Aggregates from Different Tillage and Crop Regimes. Soil Science Society of America Journal, 69(1), 141–147. https://doi.org/10.2136/sssaj2005.0141a
Zhang, G., Zeng, G., Du, C., Jiang, Y., Su, X., Xiang, R., Huang, L., Xu, M., & Zhang, C. (2007). Deposition patterns in bulk precipitation and throughfall in a subtropical mixed forest in central-south China. Forestry: An International Journal of Forest Research, 80(2), 211–221. https://doi.org/10.1093/forestry/cpm002
Yamamoto, N., Nishiura, H., Honjo, T., Ishikawa, Y., & Suzuki, K. (1995). A long-term study of atmospheric ammonia and particulate ammonium concentrations in Yokohama, Japan. Atmospheric Environment, 29(1), 97–103. https://doi.org/10.1016/1352-2310(94)00226-B
Yanagisawa, Y., & Nishimura, H. (1980). A personal sampler for measurement of nitrogen dioxide in ambient air. Journal of Japan Society of Air Pollution, 15(8), 316–323. https://doi.org/10.11298/taiki1978.15.316
Yoshikado, H. (2004). One Possible Factor Causing Recent Trend of Photochemical Oxidants. Journal of Japan Society for Atmospheric Environment / Taiki Kankyo Gakkaishi, 39(4), 188–199. https://doi.org/10.11298/taiki1995.39.4_188
Acknowledgments
This work was supported by the Kanagawa University Grant for Joint Research and JSPS KAKENHI [Grant Number JP19H00955]. We also acknowledge the Afuri Shrine for offering sampling sites and the members of our research group for their cooperation with sample collection.
Author information
Authors and Affiliations
Contributions
Yize Wang: Investigation, Formal analysis, Writing - Original Draft. Hiroshi Okochi: Investigation, Writing - Review & Editing. Manabu Igawa: Investigation, Resources, Writing - Review & Editing, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 825 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Okochi, H. & Igawa, M. Long term trends of dry and wet deposition of air pollutants at declining forest site of Mt. Oyama in Japan during 1994–2019. Water Air Soil Pollut 234, 257 (2023). https://doi.org/10.1007/s11270-023-06270-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06270-3