Abstract
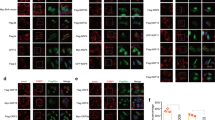
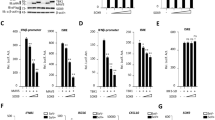
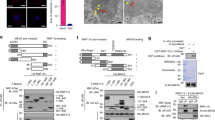
Viral infection triggers the innate antiviral immune response that rapidly produces type I interferons in most cell types to combat viruses invading. Upon viral infection, the cytoplasmic RNA sensors RIG-I/MDA5 recognize viral RNA, and then RIG-I/MDA5 is transported to mitochondria interacting with VISA through the CARD domain. From there, VISA recruits downstream antiviral signaling pathways molecules, such as TRAFs and TBK1. Eventually, IRF3 is phosphorylated and type I IFNs are induced to fight as the first line of defense against viruses. However, it remains unclear how VISA acts as a scaffold to assemble the signalosome in RIG-I-mediated antiviral signaling. Here, we demonstrated Sec13 as a novel component that was involved in VISA-mediated antiviral signaling pathway. The co-immunoprecipitation assays showed that Sec13 specifically interacts with VISA. Overexpression of Sec13 increases VISA’s aggregation and ubiquitination and significantly enhances the phosphorylation and dimerization of IRF3, facilitating the IFN-β production. Conversely, the knockdown of Sec13 attenuates Sendai virus-induced and VISA-mediated IRF3 activation and the production of IFNβ, thus weakens antiviral immune activity.






Similar content being viewed by others
References
V. Hornung, J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K.K. Conzelmann, M. Schlee, S. Endres, G. Hartmann, 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006)
O. Takeuchi, S. Akira, Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010)
B. Beutler, Z. Jiang, P. Georgel, K. Crozat, B. Croker, S. Rutschmann, X. Du, K. Hoebe, Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24, 353–389 (2006)
M. Yoneyama, T. Fujita, Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity 29, 178–181 (2008)
O. Takeuchi, S. Akira, Innate immunity to virus infection. Immunol. Rev. 227, 75–86 (2009)
M. Yoneyama, M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, T. Fujita, The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004)
M.U. Gack, Y.C. Shin, C.H. Joo, T. Urano, C. Liang, L. Sun, O. Takeuchi, S. Akira, Z. Chen, S. Inoue, J.U. Jung, TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, 916–920 (2007)
A. Pichlmair, O. Schulz, C.P. Tan, T.I. Naslund, P. Liljestrom, F. Weber, C. Reis e Sousa, RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006)
F. Ferrage, K. Dutta, E. Nistal-Villan, J.R. Patel, M.T. Sanchez-Aparicio, P. De Ioannes, A. Buku, G.G. Aseguinolaza, A. Garcia-Sastre, A.K. Aggarwal, Structure and dynamics of the second CARD of human RIG-I provide mechanistic insights into regulation of RIG-I activation. Structure 20, 2048–2061 (2012)
L.G. Xu, Y.Y. Wang, K.J. Han, L.Y. Li, Z. Zhai, H.B. Shu, VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19, 727–740 (2005)
R.B. Seth, L. Sun, C.K. Ea, Z.J. Chen, Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122, 669–682 (2005)
T. Kawai, K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K.J. Ishii, O. Takeuchi, S. Akira, IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 (2005)
E. Dixit, S. Boulant, Y. Zhang, A.S. Lee, C. Odendall, B. Shum, N. Hacohen, Z.J. Chen, S.P. Whelan, M. Fransen, M.L. Nibert, G. Superti-Furga, J.C. Kagan, Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141, 668–681 (2010)
S.M. Horner, H.M. Liu, H.S. Park, J. Briley, M. Gale Jr., Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc. Natl. Acad. Sci. USA 108, 14590–14595 (2011)
J.L. Jacobs, C.B. Coyne, Mechanisms of MAVS regulation at the mitochondrial membrane. J. Mol. Biol. 425, 5009–5019 (2013)
S.K. Saha, E.M. Pietras, J.Q. He, J.R. Kang, S.Y. Liu, G. Oganesyan, A. Shahangian, B. Zarnegar, T.L. Shiba, Y. Wang, G. Cheng, Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 25, 3257–3263 (2006)
C. Vazquez, S.M. Horner, MAVS coordination of antiviral innate immunity. J. Virol. 89, 6974–6977 (2015)
F. Hou, L. Sun, H. Zheng, B. Skaug, Q.X. Jiang, Z.J. Chen, MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011)
E.D. Tang, C.Y. Wang, MAVS self-association mediates antiviral innate immune signaling. J. Virol. 83, 3420–3428 (2009)
J.Q. He, G. Oganesyan, S.K. Saha, B. Zarnegar, G. Cheng, TRAF3 and its biological function. Adv. Exp. Med. Biol. 597, 48–59 (2007)
S. Paz, Q. Sun, P. Nakhaei, R. Romieu-Mourez, D. Goubau, I. Julkunen, R. Lin, J. Hiscott, Induction of IRF-3 and IRF-7 phosphorylation following activation of the RIG-I pathway. Cell. Mol. Biol. (Noisy-le-grand) 52, 17–28 (2006)
K.A. Fitzgerald, S.M. McWhirter, K.L. Faia, D.C. Rowe, E. Latz, D.T. Golenbock, A.J. Coyle, S.M. Liao, T. Maniatis, IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003)
S. Paz, M. Vilasco, S.J. Werden, M. Arguello, D. Joseph-Pillai, T. Zhao, T.L. Nguyen, Q. Sun, E.F. Meurs, R. Lin, J. Hiscott, A functional C-terminal TRAF3-binding site in MAVS participates in positive and negative regulation of the IFN antiviral response. Cell Res. 21, 895–910 (2011)
S. Vallabhapurapu, M. Karin, Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009)
S. Alberti, R. Halfmann, O. King, A. Kapila, S. Lindquist, A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 137, 146–158 (2009)
D. Vitour, S. Dabo, M. Ahmadi Pour, M. Vilasco, P.O. Vidalain, Y. Jacob, M. Mezel-Lemoine, S. Paz, M. Arguello, R. Lin, F. Tangy, J. Hiscott, E.F. Meurs, Polo-like kinase 1 (PLK1) regulates interferon (IFN) induction by MAVS. J. Biol. Chem. 284, 21797–21809 (2009)
B. Wu, S. Hur, How RIG-I like receptors activate MAVS. Curr. Opin. Virol. 12, 91–98 (2015)
B. Zhong, Y. Zhang, B. Tan, T.T. Liu, Y.Y. Wang, H.B. Shu, The E3 ubiquitin ligase RNF5 targets virus-induced signaling adaptor for ubiquitination and degradation. J. Immunol. 184, 6249–6255 (2010)
Y. Wang, X. Tong, X. Ye, Ndfip1 negatively regulates RIG-I-dependent immune signaling by enhancing E3 ligase Smurf1-mediated MAVS degradation. J. Immunol. 189, 5304–5313 (2012)
Y. Pan, R. Li, J.L. Meng, H.T. Mao, Y. Zhang, J. Zhang, Smurf2 negatively modulates RIG-I-dependent antiviral response by targeting VISA/MAVS for ubiquitination and degradation. J. Immunol. 192, 4758–4764 (2014)
F. You, H. Sun, X. Zhou, W. Sun, S. Liang, Z. Zhai, Z. Jiang, PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4. Nat. Immunol. 10, 1300–1308 (2009)
X. Zhou, F. You, H. Chen, Z. Jiang, Poly(C)-binding protein 1 (PCBP1) mediates housekeeping degradation of mitochondrial antiviral signaling (MAVS). Cell Res. 22, 717–727 (2012)
K. Arimoto, H. Takahashi, T. Hishiki, H. Konishi, T. Fujita, K. Shimotohno, Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc. Natl. Acad. Sci. USA 104, 7500–7505 (2007)
Y.S. Yoo, Y.Y. Park, J.H. Kim, H. Cho, S.H. Kim, H.S. Lee, T.H. Kim, Y. Sun Kim, Y. Lee, C.J. Kim, J.U. Jung, J.S. Lee, H. Cho, The mitochondrial ubiquitin ligase MARCH5 resolves MAVS aggregates during antiviral signalling. Nat. Commun. 6, 7910 (2015)
C. Castanier, N. Zemirli, A. Portier, D. Garcin, N. Bidere, A. Vazquez, D. Arnoult, MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC Biol. 10, 44 (2012)
B. Lin, Q. Ke, H. Li, N.S. Pheifer, D.C. Velliquette, D.W. Leaman, Negative regulation of the RLH signaling by the E3 ubiquitin ligase RNF114. Cytokine 99, 186–193 (2017)
J.L. Jacobs, J. Zhu, S.N. Sarkar, C.B. Coyne, Regulation of mitochondrial antiviral signaling (MAVS) expression and signaling by the mitochondria-associated endoplasmic reticulum membrane (MAM) protein Gp78. J. Biol. Chem. 289, 1604–1616 (2014)
Q. Wang, X. Liu, Y. Cui, Y. Tang, W. Chen, S. Li, H. Yu, Y. Pan, C. Wang, The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 41, 919–933 (2014)
W.W. Luo, S. Li, C. Li, Z.Q. Zheng, P. Cao, Z. Tong, H. Lian, S.Y. Wang, H.B. Shu, Y.Y. Wang, iRhom2 is essential for innate immunity to RNA virus by antagonizing ER- and mitochondria-associated degradation of VISA. PLoS Pathog. 13, e1006693 (2017)
C. Barlowe, L. Orci, T. Yeung, M. Hosobuchi, S. Hamamoto, N. Salama, M.F. Rexach, M. Ravazzola, M. Amherdt, R. Schekman, COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895–907 (1994)
J. Enninga, A. Levay, B.M.A. Fontoura, Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex. Mol. Cell. Biol. 23 (2003) 7271–7284
N.R. Salama, T. Yeung, R.W. Schekman, The Sec13p complex and reconstitution of vesicle budding from the ER with purified cytosolic proteins. EMBO J 12, 4073–4082 (1993)
L. Fu, E. Sztul, Traffic-independent function of the Sar1p/COPII machinery in proteasomal sorting of the cystic fibrosis transmembrane conductance regulator. J. Cell Biol. 160, 157–163 (2003)
V. Haucke, Vesicle budding: a coat for the COPs. Trends Cell Biol. 13, 59–60 (2003)
S.M. Stagg, C. Gurkan, D.M. Fowler, P. LaPointe, T.R. Foss, C.S. Potter, B. Carragher, W.E. Balch, Structure of the Sec13/31 COPII coat cage. Nature 439, 234–238 (2006)
S.M. Stagg, P. LaPointe, A. Razvi, C. Gurkan, C.S. Potter, B. Carragher, W.E. Balch, Structural basis for cargo regulation of COPII coat assembly. Cell 134, 474–484 (2008)
K. Matsuoka, L. Orci, M. Amherdt, S.Y. Bednarek, S. Hamamoto, R. Schekman, T. Yeung, COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93, 263–275 (1998)
L. Ellgaard, A. Helenius, ER quality control: towards an understanding at the molecular level. Curr. Opin. Cell Biol. 13, 431–437 (2001)
J.L. Brodsky, A.A. McCracken, ER protein quality control and proteasome-mediated protein degradation. Semin. Cell Dev. Biol. 10, 507–513 (1999)
K. Bienz, D. Egger, T. Pfister, Characteristics of the poliovirus replication complex. Arch. Virol. Suppl. 9, 147–157 (1994)
R.C. Rust, L. Landmann, R. Gosert, B.L. Tang, W. Hong, H.P. Hauri, D. Egger, K. Bienz, Cellular COPII proteins are involved in production of the vesicles that form the poliovirus replication complex. J. Virol. 75, 9808–9818 (2001)
J. Enninga, A. Levay, B.M. Fontoura, Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex. Mol. Cell. Biol. 23, 7271–7284 (2003)
J. Enninga, D.E. Levy, G. Blobel, B.M. Fontoura, Role of nucleoporin induction in releasing an mRNA nuclear export block. Science 295, 1523–1525 (2002)
C. von Kobbe, J.M. van Deursen, J.P. Rodrigues, D. Sitterlin, A. Bachi, X. Wu, M. Wilm, M. Carmo-Fonseca, E. Izaurralde, Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell 6, 1243–1252 (2000)
M. Ahmed, D.S. Lyles, Effect of vesicular stomatitis virus matrix protein on transcription directed by host RNA polymerases I, II, and III. J. Virol. 72, 8413–8419 (1998)
M. Capelson, Y. Liang, R. Schulte, W. Mair, U. Wagner, M.W. Hetzer, Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell 140, 372–383 (2010)
X. Niu, J. Hong, X. Zheng, D.B. Melville, E.W. Knapik, A. Meng, J. Peng, The nuclear pore complex function of Sec13 protein is required for cell survival during retinal development. J. Biol. Chem. 289, 11971–11985 (2014)
J. Zhu, T. Davoli, J.M. Perriera, C.R. Chin, G.D. Gaiha, S.P. John, F.D. Sigiollot, G. Gao, Q. Xu, H. Qu, T. Pertel, J.S. Sims, J.A. Smith, R.E. Baker, L. Maranda, A. Ng, S.J. Elledge, A.L. Brass, Comprehensive identification of host modulators of HIV-1 replication using multiple orthologous RNAi reagents. Cell Rep. 9, 752–766 (2014)
T.G. Moreira, L. Zhang, L. Shaulov, A. Harel, S.K. Kuss, J. Williams, J. Shelton, B. Somatilaka, J. Seemann, J. Yang, R. Sakthivel, D.R. Nussenzveig, A.M. Faria, B.M. Fontoura, Sec13 regulates expression of specific immune factors involved in inflammation in vivo. Sci Rep. 5, 17655 (2015)
Acknowledgements
We are grateful to Dr. Hong-Bing Shu (Medical Research Institute, Wuhan University) for his helping for providing plasmids and other reagents assistance. This work was supported by Grants from the National Natural Science Foundation of China (Grant Nos. 31370876, 31570876), the Natural Science Foundation of Jiangxi Province (20143ACB20004, 20161BAB204177), the Open Project Program of Key Laboratory of Functional Small Organic Molecule, Ministry of Education, and Jiangxi Normal University (KLFS-KF-201407), Postdoctoral Start Fund of Jiangxi Normal University (2014A).
Author information
Authors and Affiliations
Contributions
LX designed the research. TC, TX, and DW performed the experiments. LX and TC did data analysis and discussion. TC and LX wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Edited by Hartmut Hengel.
Rights and permissions
About this article
Cite this article
Chen, T., Wang, D., Xie, T. et al. Sec13 is a positive regulator of VISA-mediated antiviral signaling. Virus Genes 54, 514–526 (2018). https://doi.org/10.1007/s11262-018-1581-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-018-1581-0