Abstract
Lyme disease (LD) is a globally distributed zoonotic multisystemic condition caused by gram-negative spirochete bacteria of the Borrelia burgdorferi complex, transmitted through tick bites. Research on LD in domestic animals in Portugal is limited, potentially leading to underestimating its prevalence. This disease affects many species, including humans, making it a critical public health issue. In domestic animals, LD often presents subclinically or with non-specific clinical signs, complicating its diagnosis. Nevertheless, veterinarians should always consider LD in cases with a history of tick exposure and compatible clinical signs. Diagnostic confirmation can be achieved through serological and other complementary tests. Treatment involves eradicating the bacterial infection and managing clinical signs using a combination of antibiotics, analgesics, anti-inflammatories, and other medications. Effective prevention primarily relies on tick control measures. This review aims to provide an up-to-date state-of-the-art LD, particularly in Portugal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lyme disease (LD), or borreliosis, is a zoonotic disease, caused by Borrelia burgdorferi sensu lato, that has a worldwide distribution. It is a multisystemic disease whose clinical signs can be very unspecific, making it hard to diagnose (Baneth et al. 2016; Camire et al. 2021; Milkovičová et al. 2023). At least five genospecies are known to cause LD in humans: Borrelia afzelii, Borrelia bavariensis, Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia spielmanii (Stanek et al. 2012; Steere et al. 2016; Eisen 2020). Of these, B. afzelii, B. burgdorferi s. s., and B. garinni are considered pathogenic in dogs (Gatellet et al. 2019; ESCCAP 2023).
Lyme disease can affect a wide variety of domestic (mainly dogs and horses), wild animals and humans, having an important public health importance and reservoirs hosts can acquire this infection when exposed to infected ticks (Parry 2016; Kassab et al. 2020). Cat infection has also been reported, especially in outdoor cats, although there is less information about its specific prevalence, clinical signs and treatment (Pantchev et al. 2016; ESCCAP 2023).
Borreliosis is generally considered one of the most prevalent vector-borne diseases in Europe and North America in humans and dogs (Garrido and Borges-Costa 2018; Camire et al. 2021). In Europe, several pathogenic species of B. burgdorferi s. l. can be found. Borrelia burgdorferi s. s. is more common in Western Europe; while Borrelia lusitaniae is more frequent in Southern Europe, including the Mediterranean area(Mannelli et al. 2012).
In Portugal, LD cases must be reported for humans since 1999, but not for animals (Mannelli et al. 2012). According to INE (2020), in 2017, 20 human cases of borreliosis were reported in Portugal (seven from the north, eight from the centre, and four from Lisbon regions). Due to the biology and epidemiology of this disease, it is difficult to evaluate the real impact of this disease in the country. Moreover, there is a lack of data regarding its actual prevalence. In Portugal, the most prevalent genospecies in ticks is B. lusitaniae. However, B. afzelii, B. garinii, B. burgdorferi s. s., Borrelia valaisiana and Borrelia turdi have also been reported (Nunes et al. 2016; Núncio and Carvalho 2019).
Therefore, this review aims to summarize the information available on biology, epidemiology, clinical presentation and public health aspects of LD in companion animals, focusing on its current situation in Portugal.
Methodology
This search was carried out using Google Scholar® and Pubmed®, with the following keywords: Lyme disease; borreliosis; Portugal; dogs; cats; human. Journal articles, book chapters, and dissertations, published between 2000 and 2023 were included in this study. Moreover, official organization web pages, such as European Scientific Counsel Companion Animal Parasites (ESCCAP), Centers for Disease Control and Prevention (CDC), Companion Animal Parasite Council (CAPC), World Organization for Animal Health (WOAH), Instituto Nacional de Engenharia (INE), World Health Organization (WHO) and European Centre for Disease Prevention and Control (ECDC) were also included. Preference was given to searching for the most up-to-date evidence and Portuguese data.
Biology, transmission and pathogenesis
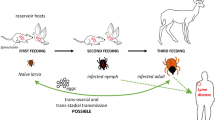
Lyme Disease is a vector-borne disease caused by gram-negative spirochete bacteria of the B. burgdorferi complex. This zoonotic disease affects multiple domestic animals, wild animals and humans (Parry 2016; Kassab et al. 2020; ESCCAP 2023). Borrelia garinii, B. turdii and B. valaisiana are primarily associated with birds, allowing transmission to long distances; while B. azfelii and B. burgdorferi s. s. are associated with mammals and B. lusitaniae with lizards (Kurtenbach et al. 2002; Norte et al. 2015). Interestingly, certain dog breeds appear to have a predisposition for infection. Adaszek et al. (2022), stated that Bernese Mountain dogs often test positive for antibodies against B. burgdorferi, indicating a higher susceptibility to infection of a hereditary infection.
Different tick species from the Ixodidae family may work as vectors of this disease (Margos et al. 2020), including Ixodes ricinus and Ixodes persulcatus (the main vectors in Europe and Asia), Ixodes scapularis and Ixodes pacificus (the main vectors in North America/USA), and Ixodes hexagonus (Stanek et al. 2012; Steere et al. 2016; Rubio and Seixas 2017). Tick larvae, nymphs and adults can become infected with these bacteria when they feed on infected hosts. For infection to occur, at least 16 to 24 h of blood meal are required (ESCCAP 2023). Vertical transmission between female dogs and puppies is also possible but uncommon (Seixas et al. 2011).
Public health importance
Vector-borne diseases pose an important threat to public health as they are responsible for more than 17% of all infectious diseases, causing more than 700,000 human deaths annually. They are becoming more and more documented globally (Alho et al. 2016; WHO 2020).
Currently, LD incidence in humans varies between 0.04 and 0.4 cases per 100.000 in Portugal (Departamento de Doenças Infeciosas do Centro de estudos de Vetores e Doenças Infeciosas Doutor Francisco Cambournac 2023).
Tick infestations are typically associated with rural areas. Roome et al. (2022), stated that people living in rural regions of the Northeastern United States, had a 33.6% higher risk of having LD compared to the urban population. However, ticks are expanding their geographical distribution due to animal migration, climate change, globalization, population growth, travel tendencies, changes in the habitat/landscapes, increasing humans’ exposure to ticks, even in urban and suburban habitats (Uspensky 2014; Rizzoli et al. 2014). In addition, an increase in outdoor activities and ongoing human exploitation of environmental resources have also increased different human-vector interactions (Dantas-Torres et al. 2012; Kilpatrick et al. 2017; Vrhovec et al. 2017; Rosà et al. 2018; Alcon-Chino and De-Simone 2022).
Cats and dogs are significant reservoirs of tick-borne diseases. Thus, preventing LD in humans and pets requires a One Health approach (Day 2011; Mencke 2013). According to published data, pet owners are more likely to have an LD diagnosis than people who do not have pets. Dogs can increase their owners’ risk of getting LD by bringing ticks home (Smith et al. 2012; Bowser and Anderson 2018; Herrin et al. 2018). Dog-to-human transmission increases as the number of dogs a person owns grows (Andersson et al. 2017).
Additionally, dogs may not show clinical signs of disease and can act as “silent reservoirs”, allowing for discreet and prolonged transmission to other animals and humans (Alho et al. 2016). On the other hand, cats have been associated with a higher risk of LD. It has been suggested that cats are at higher risk due to lower usage of anti-parasitic drugs compared to dogs, such as tick-preventive collars, and less frequent visual checks by their owners for these parasites. While screening cats for ticks may be more challenging, effective tick-checking among dog owners may have reduced the increased risk for LD associated with dog ownership in some populations (Roome et al. 2022). Nevertheless, in certain geographic regions, cats often have the freedom to come and go from our homes, bringing ticks with them. Moreover, they frequently show a higher predatory behavior, especially for small mammals, such as rodents, which are important reservoirs of ticks and Borrelia spp. (Roome et al. 2022).
Therefore, it is crucial to educate the pet owners to use effective preventive measures to prevent tick infection (in both cats and dogs). Extra attention should be given to pets during warmer seasons in both rural and urban areas.
Since companion animals are in close contact with their owners, information regarding the potential risk for the human population can be obtained by collecting ticks from pets and screening them for tick-borne diseases (Liu et al. 2019; Milkovičová et al. 2023). Research has shown that the identification of B. burgdorferi directly in dogs or in their ticks is associated with the prevalence of LD in humans in the corresponding areas (Little et al. 2010, 2021; Hendricks et al. 2017; Liu et al. 2019). The same principle applies to the recently confirmed human pathogenic tick-borne relapsing fever spirochete, Borrelia miyamotoi, which affects humans and causes B. miyamotoi disease. This bacterium is transmitted like B. burgdorferi, i.e., through tick bites (I. ricinus complex), and as of now is not known to cause disease in dogs and cats. However, systematic surveillance of ticks on domestic animals could be beneficial in assessing human exposure to infected ticks in urban areas (Liberska et al. 2023).
Healthcare professionals must make sure that patients with rash, febrile symptoms, and any possible vector exposure are evaluated for vector-borne infections and actively teach patients about the dangers of vector-borne illnesses and how to prevent them (Lee Werner et al. 2019).
Epidemiology
Borreliosis is a disease with a worldwide distribution. It is considered the most common tick-borne disease in the temperate areas of the Northern Hemisphere, and it is considered to be endemic in Central and Eastern Europe, North America and Eastern Asia (Bhate and Schwartz 2011; Kilpatrick et al. 2017; Sykes and Makiello 2017; Kassab et al. 2020). Globally, borreliosis affects 0.3 to 0.5 million people annually in the Northern Hemisphere, and only climate change will likely cause a 20% increase in LD incidence in the USA over the next ten years (Kowalec et al. 2017; Eisen 2020). Despite that, there are few published data regarding the incidence and prevalence of LD. However, medical authorities suggest that the number of cases is higher than reported (Cook and Puri 2020). The general distribution of LD is proportional to the geographical distribution of the vectors and reservoirs that are essential to the transmission of these bacteria (Margos et al. 2011).
Some authors believe that the occurrence of this disease is progressively increasing and spreading across Europe (Sykes and Makiello 2017). However, there is no regular surveillance system for LD in companion animals. Therefore, the prevalence of this disease is often inaccurate (Milkovičová et al. 2023). Moreover, free-ranging canines not receiving veterinary care have a smaller chance of being tested and considered in these databases. Additionally, there are fewer diagnostic methods available for cats. Their grooming habits and mainly indoor lifestyle suggest that they have fewer vector-borne diseases than dogs (Qurollo 2019).
Miró et al. (2022) mapped the distribution and seropositivity of dogs for specific canine vector-borne diseases using data from enzyme-linked immunosorbent assay (ELISA) tests in Europe from 2016 to 2020. The presence of B. burgdorferi antibodies was mainly concentrated in Northern and Eastern Europe. Higher rates (> 5%) were found in Austria, Czech Republic, Estonia, Finland, Germany, Lithuania, Netherlands, Norway, Poland, Slovenia, Sweden and Switzerland, while lower rates (< 1%) were registered in Andorra, Croatia, Greece, Hungary, Italy, Malta, Portugal, Romania and Spain.
There is limited information on LD in cats. To our knowledge, only three European studies have reported infection with B. burgdorferi s. l. in cats. Shaw et al. (2005) reported infection in two cats from the United UK, Tørnqvist-Johnsen et al. (2020) reported infection in another two cats from the UK, and Pantchev et al. (2016) reported infection in 6 cats from Germany and other European countries.
In the northern hemisphere, including western Europe, B. burgdorferi s. l. is transmitted mainly by I. ricinus and Portugal is considered an endemic country for this vector (Galluzzo et al. 2020; ECDC 2023).
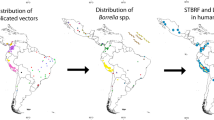
Portugal has several endemic vector-borne diseases, primarily attributed to the country’s temperate Mediterranean climate, which promotes vector production and survival (Alho et al. 2016). The studies regarding borreliosis in dogs, cats and ticks in Portugal are summarized in Table 1; Fig. 1.
Regarding dog infections, Alho et al. (2016), detected for the first time B. afzelli DNA in dogs in Portugal (3.2%), which is one of the most causative agents of tick-borne disease in Europe and North America. Maia et al. (2015) detected the first molecular evidence of naturally occurring B. burgdorferi s. l. in dogs in Portugal (0.8%). These antibodies were detected in Algarve, Bragança, Alentejo, Lisbon, North and Center Portugal using serology (Alexandre 2006; Figueiredo 2007; Cardoso et al. 2012). To our knowledge, only one study by Maia et al. (2014b) reported the prevalence of B. burgdorferi s. l. in cats in Portugal (2.2%).
Evidence shows that LD spreads geographically due to climate change (Kilpatrick et al. 2017; Garrido and Borges-Costa 2018). In recent years, several researchers have observed a rise in the prevalence of tick-borne illnesses in colder regions. This can be attributed to the increase in atmospheric temperature, which may facilitate and/or hasten the development of ticks. As a result, these vector-borne diseases may spread more widely and have a more global distribution (Rossati 2017; Rocklöv and Dubrow 2020; Rupasinghe et al. 2022). Furthermore, global warming may affect not only the survival of ticks in higher latitudes but also the host population (e.g. bird migration and rodents) and human behaviour (e.g. urban sprawl), which increases the risk of spreading this disease (Kilpatrick et al. 2017; Littman et al. 2018; Rosà et al. 2018).
Clinical presentation
Borreliosis is usually asymptomatic or has very non-specific clinical signs, making it a multisystem disease (Kassab et al. 2020; Milkovičová et al. 2023). According to Little et al. (2010), only 5% of infected dogs show clinical signs, which might be a reason to be underdiagnosed in this species.
Lyme arthritis is the most prevalent clinical sign of borreliosis in dogs, and it is usually present in young and large-breed dogs with an outdoor lifestyle (Littman et al. 2018).
Three stages of the disease can be identified in dogs: (1) early localized disease, right after the tick bite and inoculation of bacteria, when a discrete inflammatory reaction (erythema migrans) occurs on the skin, particularly around the bite; (2) early disseminated disease, characterized by disseminated erythema migrans and cranial nerve paralysis (facial nerve), meningitis and carditis; and (3) late disease, characterized by arthritis, encephalopathy, and acute polyneuropathy (Liu et al. 2019).
As previously stated, LD in dogs typically has non-specific clinical signs, which include pyrexia, lymphadenopathy, anorexia, myalgia, lethargy, lameness, diarrhea, conjunctivitis and arthritis (with the tarsus and carpus being the most usually affected joints). Less frequently, infected dogs can also show kidney failure associated with protein-losing nephropathy, myocarditis, and neurological signs with consequent behavioral changes (aggression, convulsions or facial paralysis) (Little et al. 2010; Chomel 2015; Littman et al. 2018; CAPC 2019; Galluzzo et al. 2020; ESCCAP 2023). Lyme nephritis is considered a rare condition (5–10% of cases) with a poor prognosis with fatal evolution in dogs (Borys et al. 2019).
It is unclear how LD manifests clinically in cats. Some authors state that cats are less predisposed to develop borreliosis because they are more efficient in removing ticks (Littman et al. 2018). Studies stated that experimentally infected cats did not show detectable clinical signs of disease (Lappin et al. 2015). Lobetti (2017) stated that experimentally infected cats show signs of arthritis and meningitis. However, in naturally infected cats, clinical manifestations are uncommon (ESCCAP 2023), though it is difficult to prove that clinical signs are associated with LD since Anaplasmosis has the same vector and similar clinical signs (Littman et al. 2018). There is one study that reported that one cat showed clinical signs of disease in the USA, such as lethargy, lameness, anorexia and ataxia and responded to treatment with doxycycline and another one in the UK that reports recurrent pyrexia in two cats (Shaw et al. 2005; Hoyt et al. 2018). Tørnqvist-Johnsen et al. (2020) stated that although Lyme carditis is rare, two cats positive for B. burgdorferi with serology and polymerase chain reaction (PCR) presented this condition. One of the cats also had a history of erythema migrans.
In humans, erythema migrans is the most common sign, affecting 70–80% of the patients (Schwartz et al. 2019). Erythema can be followed by other “flu-like symptoms” such as fever, headaches, myalgia, arthralgia, and fatigue. The infection can spread to other tissues and organs (nervous system, joints and skin) (Ebani et al. 2014; Chomel 2015; Stanek and Strle 2018).
Diagnosis
LD is difficult to diagnose because the clinical signs are non-specific, and subclinical infection may be present. Diagnosis should be made through a combination of tests (detection of specific antibodies and/or detection of Borrelia DNA), clinical signs, epidemiological factors, history/duration of exposure to ticks, exclusion of other diseases and response to antibiotics (Borys et al. 2019; CAPC 2019; Milkovičová et al. 2023).
Various diagnostic methods are available for LD, each with different sensitivity, specificity, and practical aspects (Table 2). Serology is considered the primary method of confirmation when LD is suspected (Kassab et al. 2020). Antibodies against this agent appear 3–5 weeks after infection and can be detected with commercial immunochromatographic tests (ESCCAP 2023). However, during the first few weeks of infection, a meager immune response can lead to false negatives (Johnson 2011). The most popular tests include IFA, ELISA and the Western Blot test. Vaccines can create antibodies that become detectable in IFA or ELISA tests, and previous exposure to the agent can also lead to false positives. The ELISA test has moderate sensitivity and specificity, allowing for the processing of multiple samples simultaneously (ESCCAP 2023 and Liu et al. 2023). However, it cannot distinguish between active infections and prior exposure or vaccination. In contrast, the Western Blot test is highly sensitive and specific, serving as a confirmatory test following a positive ELISA result. It is particularly useful in differentiating between antibodies produced due to vaccination and those from natural infection (ESCCAP 2023 and Liu et al. 2023). Western-Blot, is considered the gold standard, as it differentiates positive animals. Moreover, there are currently commercial kits that detect antibodies against the C6 synthetic peptide derived from the IR6 region within the Borrelia membrane protein VLsE, not interfering with vaccine-derived antibodies (e.g. SNAP4DXPlus IDEXX) (Littman et al. 2018). This test can also detect antibodies in cats, and it’s validated for this species because a species-cross conjugate is used (Levy et al. 2003). The SNAP 4Dx Test offers moderate sensitivity and specificity as well. It provides results within minutes and is capable of detecting antibodies to Borrelia burgdorferi and other tick-borne pathogens, making it useful for initial screening purposes (ESCCAP 2023 and Liu et al. 2023).
There are other methods of diagnosing LD, such as culture, microscopy and PCR (Table 2). However, they may not have a high sensitivity (ESCCAP 2023). Borrelia burgdorferi spirochetes are almost impossible to detect in blood smears by microscopy or Gram staining. They can be found in the soft tissues after the infection and are only temporarily in the blood. In these cases, dark field or phase contrast immunofluorescence microscopy is more accurate (Cutler et al. 2017; WOAH 2020). Observing spirochetes in synovial fluid also has low sensitivity (CAPC 2019; ESCCAP 2023). Culture is also not a highly sensitive method due to the low density and distribution of bacteria found in chronic infections (CAPC 2019; Milkovičová et al. 2023). Also, culturing Borrelia spp., may take longer than two weeks to grow and is particularly challenging. Therefore, it is neither practical nor timely to use culturing to diagnose chronic infections (Replogle et al. 2021).
PCR allows the amplification of the agent’s DNA from the dog’s fluids, tissue and tick fragments. In cats, the best material for PCR is synovial membrane, skin samples or renal biopsies. This test helps diagnose LD, and real-time quantitative PCR has improved pathogen diagnosis over the last decade (Straubinger 2000). Some authors referred to PCR as a sensitive method that can detect low quantities of organisms (Borchers et al. 2015). Therefore, it is important to note that a negative result in a PCR test does not necessarily rule out the presence of Borrelia spp. (Bergmann and Hartmann 2017). This is because the number of spirochetes in infected tissues or body fluids is often deficient. Additionally, the proper procedures for sample collection, transport, and DNA extraction are critical for obtaining reliable and consistent PCR results (Wang et al. 2010). Furthermore, the sensitivity of PCR detection varies depending on the type of specimens and the timing of collection (Marques 2015). The PCR test has variable sensitivity but high specificity. It is useful for detecting active infections and confirming diagnoses in ambiguous cases. However, its effectiveness can be limited by transient bacteremia or difficulties with sampling, such as when using synovial fluid ESCCAP 2023 and Liu et al. 2023). Furthermore, the LAMP (Loop-Mediated Isothermal Amplification) test exhibits high sensitivity and specificity and is simpler and faster than PCR. It holds potential for point-of-care testing due to its quick turnaround time and minimal equipment requirements, although it is still under research (ESCCAP 2023 and Liu et al. 2023). Lastly, Next-Generation Sequencing (NGS) provides very high sensitivity and specificity, offering detailed genetic information about strain variations. However, it is mainly used in research settings due to its complexity and cost (ESCCAP 2023 and Liu et al. 2023).
Treatment and prevention
The two main pillars of treatment can be summarized as solving the bacterial infection and managing the pain (arthritis) (Littman et al. 2018). Doxycycline (at a dose of 10 mg/kg PO or IV, SID or BID for a minimum of 1 month) is considered the first choice antibiotic. Other antibiotics in the class of tetracyclines, penicillins, macrolides and cephalosporins are also considered suitable for treatment (Littman et al. 2018; ESCCAP 2023). According to Littman et al. (2018), treatment with doxycycline is likely to be effective in cats.
In a dog with acute arthritis, antibiotic treatment should show some improvements within one to three days (Littman et al. 2018). Analgesics, such as gabapentin, non-steroidal anti-inflammatory drugs or glucocorticoids, may also be administered in the presence of neuropathic pain (Littman et al. 2018). When protein-losing nephropathy is present, diuretics, ACE inhibitors, dietary management, anticoagulants and fluid therapy can also be administered (Littman et al. 2018).
The preventative methods have been summarized in Table 3; Fig. 2. Tick control has become the method of choice (Bergmann and Hartmann 2017; Qurollo 2019; ESCCAP 2023). There are many formulations for tick control, such as collars, spot-ons and oral formulations. (Seresto®) collars (imidacloprid 10% + flumethrin 4,5%) showed to be effective for at least seven months in preventing diseases caused by the tick bite in 100% of the dogs studied by Krämer et al. (2020). Sarolaner (SimparicaⓇ) and afoxolaner (NexguardⓇ) also prevent tick infections (Six et al. 2016). It is important to note that topical permethrins should not be applied to cats (Littman et al. 2018).
In addition to the use of effective ectoparasiticides, inspecting and brushing the animals’ coats in the areas where ticks have excellent affinity (limbs, ears, muzzle and corners of the eyes), cutting vegetation or grass in the pets’ habitat and rodent control can be measures to control ticks and also wild animals that are infested with ticks (CAPC 2019; WOAH 2020; Nascimento and Barros 2023).
Some vaccines are also available for dogs to control B. burgdorferi, but their efficacy is still unclear, and some only protect against B. burgdorferi s. s. (Littman et al. 2018; Stillman et al. 2019; ESCCAP 2023).
Advisory boards encourage all dogs getting veterinary care to undergo a yearly screening for vector-borne diseases to promote early diagnosis, treatment, and prevention (Creevy et al. 2019).
Conclusion
LD has been a concern in Europe for several decades due to change in its distribution and prevalence. The spread of this disease and others to new areas is influenced by traveling and migration of people and animals, climate change, and the importation of animals from endemic areas. Close contact between animals and humans increases the risk of transmitting pathogenic diseases such as LD. Thus, it is important to perform diagnostic tests whenever there is a history of being exposed to ticks and/or showing clinical symptoms compatible with the disease. It is also important to educate pet owners about the significance of tick control in dogs and cats to prevent the transmission of tick-borne diseases.
Therefore, One Health and transdisciplinary approach is crucial to reducing the incidence of this zoonosis. Cooperation between veterinarians, medical doctors, public health agents, and other health professionals is crucial to prevent, monitor and manage future cases and outbreaks of LD in different hosts.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- BID:
-
Bis in die (twice a day)
- CAPC:
-
Companion Animal Parasite Council
- CDC:
-
Centers for Disease Control and Prevention
- CVBD:
-
Canine Vector-borne diseases
- ECDC:
-
European Centre for Disease Prevention and Control
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- ESCCAP:
-
European Scientific Counsel Companion Animal Parasites
- IFA:
-
Indirect Immunofluorescence
- INE:
-
Institudo Nacional de Estatística
- IV:
-
Intravenous
- LAMP:
-
Loop-Mediated Isothermal Amplification
- NGS:
-
Next-Generation Sequencing
- PCR:
-
polymerase chain reaction
- PO:
-
Per os (orally)
- SID:
-
Semel in die (once a day)
- UK:
-
United Kingdom
- USA:
-
United States of America
- WHO:
-
World Health Organization
- WOAH:
-
World Organization for Animal Health
References
Adaszek Ł, Pisarek M, Kalinowski M et al (2022) Lyme disease in Bernese Mountain Dogs. Is it a real problem? Pol J Vet Sci 25:639–647. https://doi.org/10.24425/pjvs.2022.142036
Alcon-Chino MET, De-Simone SG (2022) Recent advances in the immunologic method applied to tick-borne diseases in Brazil. Pathogens 11:870. https://doi.org/10.3390/pathogens11080870
Alexandre N (2006) Estudo clínico e epidemiológico da febre botonosa, ehrlichiose canina e borreliose de Lyme numa população de canídeos domésticos do Algarve. Dissertation, Technical University of Lisbon
Alho AM, Pita J, Amaro A et al (2016) Seroprevalence of vector-borne pathogens and molecular detection of Borrelia afzelii in military dogs from Portugal. Parasit Vectors 9:225. https://doi.org/10.1186/s13071-016-1509-2
Andersson MO, Tolf C, Tamba P et al (2017) Canine tick-borne diseases in pet dogs from Romania. Parasit Vectors 10:155. https://doi.org/10.1186/s13071-017-2092-x
Baneth G, Nachum-Biala Y, Halperin T et al (2016) Borrelia persica infection in dogs and cats: clinical manifestations, clinicopathological findings and genetic characterization. Parasit Vectors 9:244. https://doi.org/10.1186/s13071-016-1530-5
Baptista S, Quaresma A, Aires T et al (2004) Lyme borreliosis spirochetes in questing ticks from mainland Portugal. Int J Med Microbiol Suppl 293:109–116. https://doi.org/10.1016/S1433-1128(04)80016-0
Bergmann M, Hartmann K (2017) Vector-borne diseases in cats in Germany. Tierarztl Prax Ausg K Kleintiere Heimtiere 45:329–335. https://doi.org/10.15654/TPK-160874
Bhate C, Schwartz RA (2011) Lyme disease. J Am Acad Dermatol 64:619–636. https://doi.org/10.1016/j.jaad.2010.03.046
Borchers AT, Keen CL, Huntley AC, Gershwin ME (2015) Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun 57:82–115. https://doi.org/10.1016/j.jaut.2014.09.004
Borys MA, Kass PH, Mohr FC, Sykes JE (2019) Differences in clinicopathologic variables between Borrelia C6 antigen seroreactive and Borrelia C6 seronegative glomerulopathy in dogs. J Vet Intern Med 33:2096–2104. https://doi.org/10.1111/jvim.15586
Bowser NH, Anderson NE (2018) Dogs (Canis familiaris) as sentinels for human infectious disease and application to Canadian populations: a systematic review. Vet Sci 5:83. https://doi.org/10.3390/VETSCI5040083
Camire AC, Hatke AL, King VL et al (2021) Comparative analysis of antibody responses to outer surface protein (osp)A and OspC in dogs vaccinated with Lyme disease vaccines. Vet J 273:105676. https://doi.org/10.1016/j.tvjl.2021.105676
CAPC (2019) Lyme Disease. https://capcvet.org/guidelines/lyme-disease/. Accessed 30 Aug 2023
Cardoso L, Mendão C, Madeira de Carvalho L (2012) Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal - a national serological study. Parasit Vectors 5:62. https://doi.org/10.1186/1756-3305-5-62
Carvalho IL, De, Milhano N, Santos AS et al (2008) Detection of Borrelia lusitaniae, Rickettsia sp. IRS3, Rickettsia monacensis, and Anaplasma phagocytophilum in Ixodes ricinus Collected in Madeira Island, Portugal. Vector Borne Zoonotic Dis 8:575–580. https://doi.org/10.1089/vbz.2007.0245
Chomel B (2015) Lyme disease. Rev Sci Tech 34:569–576. https://doi.org/10.20506/RST.34.2.2380
Cook MJ, Puri BK (2020) Estimates for Lyme borreliosis infections based on models using sentinel canine and human seroprevalence data. Infect Dis Model 5:871–888. https://doi.org/10.1016/j.idm.2020.10.004
Creevy KE, Grady J, Little SE et al (2019) 2019 AAHA Canine Life Stage guidelines. J Am Anim Hosp Assoc 55:267–290. https://doi.org/10.5326/JAAHA-MS-6999
Cutler SJ, Rudenko N, Golovchenko M et al (2017) Diagnosing borreliosis. Vector Borne Zoonotic Dis 17:2–11. https://doi.org/10.1089/vbz.2016.1962
Dantas-Torres F, Chomel BB, Otranto D (2012) Ticks and tick-borne diseases: a one health perspective. Trends Parasitol 28:437–446. https://doi.org/10.1016/J.PT.2012.07.003
Day MJ (2011) One health: the importance of companion animal vector-borne diseases. Parasit Vectors 4:49. https://doi.org/10.1186/1756-3305-4-49
Departamento de Doenças Infeciosas do Centro de estudos de Vetores e Doenças Infeciosas Doutor Francisco Cambournac (2023) REVIVE 2022 Culicídeos e Ixodídeos. http://hdl.handle.net/10400.18/8611. Accessed 21 Apr 2024
Dietrich F, Schmidgen T, Maggi RG et al (2010) Prevalence of Bartonella henselae and Borrelia burgdorferi Sensu Lato DNA in Ixodes ricinus ticks in Europe. Appl Environ Microbiol 76:1395–1398. https://doi.org/10.1128/AEM.02788-09
Ebani VV, Bertelloni F, Torracca B, Cerri D (2014) Serological survey of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, and Ehrlichia canis infections in rural and urban dogs in Central Italy. Ann Agric Environ Med 21:671–675. https://doi.org/10.5604/12321966.1129912
ECDC (2023) Ixodes ricinus - current known distribution: August 2023. https://www.ecdc.europa.eu/en/publications-data/ixodes-ricinus-current-known-distribution-august-2023. Accessed 12 Oct 2023
Eisen L (2020) Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: a review. Ticks Tick Borne Dis 11:101359. https://doi.org/10.1016/J.TTBDIS.2019.101359
ESCCAP (2023) Control of vector-borne diseases in dogs and cats. https://www.esccap.org/uploads/docs/5y4xn3fr_0775_ESCCAP_Guideline_GL5_20221228_1p.pdf. Accessed 14 Aug 2023
Figueiredo T (2007) Estudo da Prevalência de Doenças Associadas a Vectores em Canídeos Domésticos do Distrito de Bragança. Dissertation, University of Lisbon
Galluzzo P, Grippi F, Di Bella S et al (2020) Seroprevalence of Borrelia burgdorferi in stray dogs from Southern Italy. Microorganisms 8:1688. https://doi.org/10.3390/microorganisms8111688
Garrido PM, Borges-Costa J (2018) Doença de Lyme: Epidemiologia e Manifestações Clínicas Cutâneas. Port J Dermatol Venerol 76:169–176. https://doi.org/10.29021/spdv.76.2.907
Gatellet M, Vanderheyden S, Abee M et al (2019) A suspected case of Lyme Borreliosis in a dog from Belgium. Case Rep Vet Med 2019:1–3. https://doi.org/10.1155/2019/3973901
Hendricks B, Mark-Carew M, Conley J (2017) Evaluating the utility of companion animal tick surveillance practices for monitoring spread and occurrence of human Lyme disease in West Virginia, 2014–2016. Geospat Health 12:309–315. https://doi.org/10.4081/gh.2017.582
Herrin BH, Beall MJ, Feng X et al (2018) Canine and human infection with Borrelia burgdorferi in the New York City metropolitan area. Parasit Vectors 11:187. https://doi.org/10.1186/s13071-018-2774-z
Hoyt K, Chandrashekar R, Beall M et al (2018) Evidence for clinical anaplasmosis and borreliosis in cats in Maine. Top Companion Anim Med 33:40–44. https://doi.org/10.1053/j.tcam.2018.05.002
INE (2020) Estatística da Saúde: 2018. https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=257793024&PUBLICACOESmodo=2. Accessed 17 Oct 2023
Johnson BJB (2011) Laboratory diagnostic testing for Borrelia burgdorferi infection. Lyme disease: an evidence-based approach. CABI, Wallingford, pp 73–88
Kassab S, Dankar E, Pereira J et al (2020) Borreliose canina. Enciclopédia Biosfera 17:160–178. https://doi.org/10.18677/EnciBio_2020B13
Kilpatrick AM, Dobson ADM, Levi T et al (2017) Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Philos Trans R Soc B: Biol Sci 372:20160117. https://doi.org/10.1098/rstb.2016.0117
Kowalec M, Szewczyk T, Welc-Falȩciak R et al (2017) Ticks and the city - are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasit Vectors 10:573. https://doi.org/10.1186/s13071-017-2391-2
Krämer F, Hüsken R, Krüdewagen EM et al (2020) Prevention of transmission of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum by Ixodes spp. ticks to dogs treated with the Seresto® collar (imidacloprid 10% + flumethrin 4.5%). Parasitol Res 119:299–315. https://doi.org/10.1007/S00436-019-06394-8
Kurtenbach K, De Michelis S, Etti S et al (2002) Host association of Borrelia burgdorferi sensu lato– the key role of host complement. Trends Microbiol 10:74–79. https://doi.org/10.1016/S0966-842X(01)02298-3
Lappin MR, Chandrashekar R, Stillman B et al (2015) Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats after exposure to wild-caught adult Ixodes scapularis. J Vet Diagn Invest 27:522–525. https://doi.org/10.1177/1040638715593598
Lee Werner S, Kirthi Banda B, Lee Burnsides C, James Stuber A (2019) Zoonosis: update on existing and emerging Vector-Borne illnesses in the USA. Curr Emerg Hosp Med Rep 7:91–106. https://doi.org/10.1007/s40138-019-00189-y
Levy SA, O’Connor TP, Hanscom JL, Shields P (2003) Evaluation of a canine C6 ELISA Lyme disease test for the determination of the infection status of cats naturally exposed to Borrelia burgdorferi. Vet Ther 4:172–177
Liberska JA, Michalik JF, Dabert M (2023) Exposure of dogs and cats to Borrelia miyamotoi infected Ixodes ricinus ticks in urban areas of the city of Poznań, west-central Poland. Ticks Tick Borne Dis 14:102188. https://doi.org/10.1016/j.ttbdis.2023.102188
Little SE, Heise SR, Blagburn BL et al (2010) Lyme borreliosis in dogs and humans in the USA. Trends Parasitol 26:213–218. https://doi.org/10.1016/J.PT.2010.01.006
Little S, Braff J, Place J et al (2021) Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2013–2019. Parasit Vectors 14(10). https://doi.org/10.1186/s13071-020-04514-3
Littman MP, Gerber B, Goldstein RE et al (2018) ACVIM consensus update on Lyme borreliosis in dogs and cats. J Vet Intern Med 32:887–903. https://doi.org/10.1111/jvim.15085
Liu Y, Nordone SK, Yabsley MJ et al (2019) Quantifying the relationship between human Lyme disease and Borrelia burgdorferi exposure in domestic dogs. Geospat Health 14:111–120. https://doi.org/10.4081/gh.2019.750
Liu Q, Jin X, Cheng J et al (2023) Advances in the application of molecular diagnostic techniques for the detection of infectious disease pathogens (review). Mol Med Rep 27:104. https://doi.org/10.3892/mmr.2023.12991
Maia C, Ferreira A, Nunes M et al (2014a) Molecular detection of bacterial and parasitic pathogens in hard ticks from Portugal. Ticks Tick Borne Dis 5:409–414. https://doi.org/10.1016/j.ttbdis.2014.01.009
Maia C, Ramos C, Coimbra M et al (2014b) Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit Vectors 7:115. https://doi.org/10.1186/1756-3305-7-115
Maia C, Almeida B, Coimbra M et al (2015) Bacterial and protozoal agents of canine vector-borne diseases in the blood of domestic and stray dogs from southern Portugal. Parasit Vectors 8:138. https://doi.org/10.1186/s13071-015-0759-8
Mannelli A, Martello E, Tomassone L, et al (2012) Inventory of available data and data sources and proposal for data collection on vector-borne zoonoses in animals. EFSA Supporting Publications 9:EN-234. https://doi.org/10.2903/sp.efsa.2012.EN-234
Margos G, Vollmer SA, Ogden NH, Fish D (2011) Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect Genet Evol 11:1545–1563. https://doi.org/10.1016/J.MEEGID.2011.07.022
Margos G, Fedorova N, Becker NS et al (2020) Borrelia maritima sp. Nov., a novel species of the borrelia burgdorferi sensu lato complex, occupying a basal position to north American species. Int J Syst Evol Microbiol 70:849–856. https://doi.org/10.1099/ijsem.0.003833
Marques AR (2015) Laboratory diagnosis of Lyme Disease - advances and challenges. Infect Dis Clin North Am 29:295–307. https://doi.org/10.1016/j.idc.2015.02.005
Mencke N (2013) Future challenges for parasitology: vector control and ‘One health’ in Europe. Vet Parasitol 195:256–271. https://doi.org/10.1016/j.vetpar.2013.04.007
Milhano N, de Carvalho IL, Alves AS et al (2010) Coinfections of Rickettsia slovaca and Rickettsia helvetica with Borrelia lusitaniae in ticks collected in a Safari Park, Portugal. Ticks Tick Borne Dis 1:172–177. https://doi.org/10.1016/j.ttbdis.2010.09.003
Milkovičová M, Šimková J, Valko-Rokytovská M et al (2023) Lyme borreliosis in dogs: background, epidemiology, diagnostics, treatment and prevention. Folia Vet 67:75–90. https://doi.org/10.2478/fv-2023-0009
Miró G, Wright I, Michael H et al (2022) Seropositivity of main vector-borne pathogens in dogs across Europe. Parasit Vectors 15:189. https://doi.org/10.1186/s13071-022-05316-5
Nascimento LD do, de Barros B (2023) Borrelia em canídeos: Revisão. Pubvet 17:e1392. https://doi.org/10.31533/pubvet.v17n5e1392
Norte AC, Alves da Silva A, Alves J et al (2015) The importance of lizards and small mammals as reservoirs for Borrelia lusitaniae in Portugal. Environ Microbiol Rep 7:188–193. https://doi.org/10.1111/1758-2229.12218
Núncio MS, Carvalho IL (2019) Borreliose de Lyme. In: Núncio MS, Alves MJ (eds) Doenças Associadas a Artrópodes Vetores e Roedores, 2nd edn. Instituto Nacional de Saúde Doutor Ricardo Jorge, Lisboa, pp 99–110
Nunes M, Parreira R, Maia C et al (2016) Molecular identification of Borrelia Genus in questing hard ticks from Portugal: phylogenetic characterization of two novel relapsing fever-like Borrelia Sp. Infect Genet Evol 40:266–274. https://doi.org/10.1016/j.meegid.2016.03.008
Pantchev N, Vrhovec MG, lobokar, Pluta S, Straubinger RK (2016) Seropositivity of Borrelia burgdorferi in a cohort of symptomatic cats from Europe based on a C6-peptide assay with discussion of implications in disease aetiology. Berl Munch Tierarztl Wochenschr 129:333–339
Parry N (2016) Canine borreliosis: epidemiology, pathogenesis, clinical signs, and diagnostics. Companion Anim 21:323–331. https://doi.org/10.12968/COAN.2016.21.6.323
Pereira A, Parreira R, Cotão AJ et al (2018) Tick-borne bacteria and protozoa detected in ticks collected from domestic animals and wildlife in central and southern Portugal. Ticks Tick Borne Dis 9:225–234. https://doi.org/10.1016/j.ttbdis.2017.09.008
Qurollo B (2019) Feline vector-borne diseases in North America. Vet Clin Small Anim 49:687–702. https://doi.org/10.1016/j.cvsm.2019.02.012
Replogle AJ, Sexton C, Young J et al (2021) Isolation of Borrelia miyamotoi and other Borreliae using a modified BSK medium. Sci Rep 11:1926. https://doi.org/10.1038/s41598-021-81252-1
Rizzoli A, Silaghi C, Obiegala A et al (2014) Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health 2:251. https://doi.org/10.3389/fpubh.2014.00251
Rocklöv J, Dubrow R (2020) Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol 21:479–483. https://doi.org/10.1038/s41590-020-0648-y
Roome A, Wander K, Garruto RM (2022) Cat ownership and rural residence are associated with Lyme disease prevalence in the Northeastern United States. Int J Environ Res Public Health 19:5618. https://doi.org/10.3390/ijerph19095618
Rosà R, Andreo V, Tagliapietra V et al (2018) Effect of climate and land use on the spatio-temporal variability of tick-borne bacteria in Europe. Int J Environ Res Public Health 15:732. https://doi.org/10.3390/ijerph15040732
Rossati A (2017) Global Warming and its health impact. Int J Occup Environ Med 8:7–20
Rubio KAJ, Seixas FAV (2017) Revisão de Literatura Borrelia burgdorferi em canídeos. Rev Ci 4:115–121
Rupasinghe R, Chomel BB, Martínez-López B (2022) Climate change and zoonoses: a review of the current status, knowledge gaps, and future trends. Acta Trop 226:106225. https://doi.org/10.1016/j.actatropica.2021.106225
Schwartz AM, Hinckley AF, Mead PS et al (2019) Surveillance for Lyme Disease — United States, 2008–2015. MMWR Surveillance Summaries 66:1–12. https://doi.org/10.15585/MMWR.SS6622A1
Seixas R, Alho AM, Guerra D, Carvalho LM (2011) Doenças caninas de transmissão vectorial: uma picada com muitas consequências! Vet Med 13:18–36
Shaw SE, Binns SH, Birtles RJ et al (2005) Molecular evidence of tick-transmitted infections in dogs and cats in the United Kingdom. Vet Rec 157:645–648. https://doi.org/10.1136/vr.157.21.645
Six RH, Young DR, Myers MR, Mahabir SP (2016) Comparative speed of kill of sarolaner (Simparica™) and afoxolaner (NexGard®) against induced infestations of Ixodes scapularis on dogs. Parasit Vectors 9:79. https://doi.org/10.1186/s13071-016-1307-x
Smith FD, Ballantyne R, Morgan ER, Wall R (2012) Estimating Lyme disease risk using pet dogs as sentinels. Comp Immunol Microbiol Infect Dis 35:163–167. https://doi.org/10.1016/j.cimid.2011.12.009
Stanek G, Strle F (2018) Lyme borreliosis–from tick bite to diagnosis and treatment. FEMS Microbiol Rev 42:233–258. https://doi.org/10.1093/femsre/fux047
Stanek G, Wormser GP, Gray J, Strle F (2012) Lyme borreliosis. Lancet 379:461–473. https://doi.org/10.1016/S0140-6736(11)60103-7
Steere AC, Strle F, Wormser GP et al (2016) Lyme borreliosis. Nat Rev Dis Primers 2:16090. https://doi.org/10.1038/nrdp.2016.90
Stillman BA, Thatcher B, Beall MJ et al (2019) Borrelia burgdorferi antibody test results in dogs administered 4 different vaccines. Top Companion Anim Med 37:100358. https://doi.org/10.1016/J.TCAM.2019.100358
Straubinger RK (2000) PCR-Based quantification of Borrelia burgdorferi organisms in Canine Tissues over a 500-Day postinfection period. J Clin Microbiol 38:6. https://doi.org/10.1128/JCM.38.6.2191-2199.2000
Sykes RA, Makiello P (2017) An estimate of Lyme borreliosis incidence in Western Europe†. J Public Health (Oxf) 39:74–81. https://doi.org/10.1093/PUBMED/FDW017
Tørnqvist-Johnsen C, Dickson SA, Rolph K et al (2020) First report of Lyme borreliosis leading to cardiac bradydysrhythmia in two cats. JFMS Open Rep 6. https://doi.org/10.1177/2055116919898292
Uspensky I (2014) Tick pests and vectors (Acari: Ixodoidea) in European towns: introduction, persistence and management. Ticks Tick Borne Dis 5:41–47. https://doi.org/10.1016/J.TTBDIS.2013.07.011
Vrhovec MG, Pantchev N, Failing K et al (2017) Retrospective analysis of Canine Vector-borne diseases (CVBD) in Germany with emphasis on the endemicity and risk factors of Leishmaniosis. Parasitol Res 116:131–144. https://doi.org/10.1007/S00436-017-5499-6
Wang G, Aguero-Rosenfeld A, Wormser G et al (2010) Detection of Borrelia burgdorferi. In: Samuels D, Radolf J (eds) Borrelia. Molecular biology, host interaction, and pathogenesis. Caister Academic, pp 279–298
WHO (2020) Vector-borne diseases. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. Accessed 14 Apr 2024
WOAH (2020) Borrelia spp. (Infection with). https://www.woah.org/app/uploads/2022/02/borrelia-spp-infection-with.pdf. Accessed 17 Oct 2023
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by internal funds provided by the Faculty of Veterinary Medicine, Lusófona University - University Center of Lisbon, through the project DOLYSUB (2023–2024). CJB would also like to thank Fundação para a Ciência e Tecnologia (FCT) for the support through CITAB (https://doi.org/10.54499/UIDB/04033/2020) and CiiEM (https://doi.org/10.54499/UIDB/04585/2020).
Author information
Authors and Affiliations
Contributions
RP: Conceptualization, searching databases and writing-original draft preparation. CJB: Conceptualization, searching database, writing-original draft preparation and writing-review and editing. SM: Conceptualization. AM: Conceptualization. JF: Conceptualization, methodology and writing-review and editing. AB: Conceptualization, methodology, supervision and writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Picado, R., Baptista, C.J., Meneses, A. et al. Lyme disease in companion animals: an updated state-of-art and current situation in Portugal. Vet Res Commun (2024). https://doi.org/10.1007/s11259-024-10532-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11259-024-10532-8