Abstract
Unlike trees, shrubs (i.e., multiple-stemmed woody plants) do not need evenly spaced large diameter structural roots and therefore should be more responsive to heterogeneous distributions of soil resources and spread further per unit belowground biomass. We therefore hypothesized that compared to trees, shrubs respond more to asymmetric distributions of nutrients, reach nutrient-rich patches of soil faster, and do so with less below-ground biomass. To test these three hypotheses, we planted individual seedlings of shrubs (Cornus racemosa, Rhus glabra, and Viburnum dentatum) and trees (Acer rubrum, Betula populifolia, and Fraxinus americana) in the centers of sand-filled rectangular boxes. In one direction we created a stepwise gradient of increasing nutrients with slow-release fertilizer; in the other direction, no fertilizer was added. Seedlings were harvested when their first root reached the plexiglass-covered fertilized end of their box; time taken, above-ground biomass, and below-ground biomass per nutrient segment were determined. Shrubs and trees did not consistently differ in precision of root foraging (i.e., the ratio of biomass in the fertilized and unfertilized soil) or in rates (g/day) and efficiencies (cm/day) of lateral root growth. Interspecific variation appeared more related to species’ habitats than to growth form. The fastest and most efficient roots were produced by the shrub (R. glabra) and the tree (B. populifolia), both characteristic of poor and heterogeneous soils. Root foraging by R. glabra was also facilitated by rapid rhizomatous expansion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In temporarily and spatially heterogeneous soils, competitive benefits are likely to accrue to plants that quickly discover and then exploit resource-rich patches (Freschet et al. 2021; Lynch 2022). In soil volumes in which roots are already present and resources suddenly become available, attention is warranted to rates of proliferation of roots or mycorrhizal hyphae (van der Heijden et al. 2015), as well as to rates of exudate production (Wen et al. 2022) and changes in nutrient uptake kinetics (White et al. 2013). In addition to rates of proliferation, many studies of root behavior compare species on the basis of their preferential root production in nutrient-rich patches of soil as well as in the degree to which they dominate these rich patches (i.e., precision and scale, sensu Campbell et al. 1991). Here, we address the much less studied issue of relative rates of belowground resource patch discovery (but see de Kroon and Mommer 2006) with an experimental comparison of three species of trees and three species of shrubs.
Initially root-free and resource-rich volumes of soil available for discovery by exploratory roots occur naturally as well as due to human actions. On rockslides, rock outcrops, lava flows, newly deposited sediments, and other sites where primary succession is underway, there are often only pockets of soil with water and nutrients available to colonizing roots (Fitzsimons and Michael 2017). Where these soil pockets are colonized vegetatively rather than by seed, the colonizing roots or root-producing stems sometimes must first cross inhospitable terrain such as bare rock. For example, roots of hemi-epiphytic figs (Ficus spp.) must grow up tree stems to colonize nutrient-rich epiphytic soils (Putz and Holbrook 1989). Root gaps in established vegetation, the belowground equivalent of canopy gaps, also are open for colonization after they are variously created such as by excavating animals and uprooting trees (Sanford 1989; Wilczynski and Pickett 1993; Ostertag 1998). Knowledge about which plants most rapidly colonize newly enriched soil volumes could be useful for ecosystem management (Lynch 2022; Jing et al. 2022). For instance, differential root colonization rates could help explain differences in the competitive abilities of plants of different growth forms such as why shrubs are especially potent competitors with trees where soils are heterogeneous, nutrient-poor, and severely drained (Putz and Canham 1992; Montgomery et al. 2010).
Shrubs have many characteristics that should favor rapid soil exploration. Like lianas, which were recently shown to consistently reach nutrient baits more quickly than trees (Putz 2023), most shrub stems are slender (Larjavaara 2015) and hence do not need large diameter structural roots (Götmark et al. 2016) nor do they need to distribute roots evenly around their stems for biomechanical purposes; these characteristics should increase the soil exploration capacities of shrubs per unit biomass invested below-ground. Also like many lianas (Mori et al. 2021), shrubs vegetatively produce new stems from roots and rhizomes, thus providing a further benefit from rapid and efficient lateral growth below-ground.
In this greenhouse study, we compare trees and shrubs on the basis of their belowground responses to a nutrient gradient. Based on characteristics of the shrub growth form described above, we expected that compared to trees, shrubs produce more spatially asymmetric distributions of roots in response to asymmetries in nutrient availability. We also expected that this architectural versatility, coupled with lack of thick roots, helps shrubs more rapidly colonize nutrient-rich volumes of soil and to do so with less belowground biomass than trees.
Materials and methods
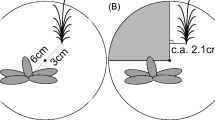
This experiment was conducted with 1 year-old seedlings in a greenhouse in Millbrook, New York (41o50′N, 73°, 45′W). Randomly selected bare-root seedlings of trees (Acer rubrum, Betula populifolia, and Fraxinus americana) and shrubs (Cornus racemosa, Rhus glabra, and Viburnum dentatum) purchased from a local nursery (N = 8 per species) were planted in early June in the centers of 60 × 15 × 40 cm (length x breadth x depth) plywood boxes filled with washed sand over a 2 cm thick layer of coarse quartz gravel (Fig. 1). One end of each box was made from plexiglass that was kept dark other than when checking for roots. Between each seedling and the root observation window, we created a four-step gradient of increasing nutrient availability by adding increasing amounts of a complete nutrient slow-release fertilizer (Sierra Chemical Company, California) to the sand when the boxes were being filled. Starting 6 cm from the plant the 6 cm wide (3600 cm3) bands of sand contained 1, 2, 4, and 8 g of fertilizer which corresponds with 7.5, 15, 30, and 60% of the recommended quantity for sensitive nursery stock. In the opposite direction, we added no fertilizer. The plants were irrigated twice weekly or as needed.
Plants were grown until the first root reached the observation window (range = 19–182 d) at which time the entire plant was harvested. Final sample sizes ranged from 4 to 8 plants per species due to mortality. Roots were harvested by section from the soil fertility gradient by cutting down through the soil with a sharpened blade. Roots were removed from the soil by hand followed by washing in a Gillison Hydropneumatic Elutriation System. Roots and shoots were then dried to constant weight at 80°C and weighed. Initial plant biomass and root: shoot biomass ratios were estimated using an additional eight randomly selected seedlings per species from the original planting stock.
Differences between trees and shrubs were examined using analysis of variance (PROC GLM in SAS; SAS Institute 1987), with species nested within growth form. The response variables tested were the ratios of root mass in the fertilized and unfertilized soil (fertilized unfertilized−1), overall root and shoot growth rates (g day−1), root: shoot biomass ratios (g g−1), rates of lateral root growth up the nutrient gradient (cm day−1), and efficiency of this lateral growth as measured in root mass used to grow the 24 cm to the end wall (cm g−1). Because species are the true level of replication for tests of the hypothesis that the two growth forms differ, F statistics for effects of growth form were computed using the growth form * species interaction term as the error mean square. Since the effects of growth form were not significant for any of the dependent variables, further analyses were conducted to test for differences among the 6 species combined (simple 1-way analysis of variance, with species as a random effect, using PROC GLM in SAS). Pairwise differences in species means were tested using Tukey’s Studentized Range Test at P < 0.05 to control experiment-wise error.
The species used in this experiment overlap considerably in ecological ranges and are frequently sympatric in southern New England. C. racemosa Lam. is a clonal shrub that grows to be 2–3 m tall and forms dense, laterally spreading clumps. V. dentatum L. is similar in growth form to C. racemosa but generally does not grow to be as tall nor are its clonal clumps as dense; both species are characteristic of mesic, nutrient-rich, alluvial soils, but C. racemosa also occurs on poorer sites. R. glabra L., the third shrub species in the experiment, forms larger and more diffuse clonal clumps in drier and more nutrient-poor sites than the other two species. All three tree species used (A. rubrum L., F. americana L., and B. populifolia Marsh) are fairly light-demanding and geographically widespread, but in our study area B. populifolia is characteristic of rocky areas with nutrient-poor and severely drained soils. All three tree species stump sprout but, unlike the studied shrubs, do not spread clonally.
Results and discussion
Contrary to our predictions there were no consistent differences between the shrubs and trees in the ratio of root mass in the fertilized and unfertilized soil, lateral root growth efficiency, root or shoot growth rates, or root: shoot ratio (Table 1, Fig. 2). All six species generally produced more roots in the fertilized than in the unfertilized portions of the boxes (Fig. 2a), but only R. glabra showed this tendency to a substantial degree. Note that this parameter is not equivalent to root foraging precision (sensu Campbell et al. 1991) because the plants were destructively harvested when the first root reached the end wall, which precluded root proliferation in the nutrient-rich patch. Lateral root growth efficiency up the nutrient gradient (cm/g) was quite variable among species (Fig. 2b) but R. glabra was again the most efficient; the other two shrub species were markedly less efficient than any of the tree species tested. Among the trees, A. rubrum roots reached the nutrient-rich end of the planting boxes with smaller investments in root biomass than observed in the other two species (Fig. 2b). R. glabra and B. populifolia grew most rapidly up the nutrient gradient (Fig. 2c) and grew roots the fastest (Fig. 2d); roots of four R. glabra and two B. populifolia seedlings reached the observation window within about 3 weeks. Shoot growth rates by R. glabra were markedly higher than in any other tree or shrub species (Fig. 2e). Among the three shrubs tested, the species with the highest root foraging efficiency and most rapid lateral root growth rate (R. glabra) also had the lowest root/shoot ratio (Fig. 2f). In contrast, a tree with rapid root growth toward the nutrient-rich bait (B. populifolia) had a high mean root/shoot ratio compared with the other tree species tested. These findings suggest that root-shoot ratios are not good predictors of root foraging efficiency.
Growth characteristics of seedlings planted in the middles of sand-filled boxes with no fertilizer added to one side and a gradient of increasing fertilizer added to the other (N = 4−8 per species). Error bars are ± 1 S. E. of the mean. Species with different letters differ at p < 0.05 based on Tukey’s Studentized Range Tests. A. Ratio of root plus rhizome biomass in the fertilized and unfertilized sides of the planting boxes. B. Maximum lateral extension of the root system per gram of root tissue in the fertilized side of the boxes. C. Below-ground growth rates (cm/day) up the fertility gradient. D. Total below-ground growth rate (g/day). E. Above-ground growth rate (g/day). F. The ratio of below-ground to above-ground biomass (including initial biomass). The three species in the left half of each box are trees (FRAM Fraxinus americana, ACRU Acer rubrum, BEPO Betula populifolia) while those on the right are shrubs (VIDE Viburnum dentatum, CORA Cornus racemosa, and RHGL Rhus glabra)
Root systems of species with high rates of growth along the soil fertility gradient (i.e., R. glabra and B. populifolia) tended not to proliferate in low the fertility soil (Fig. 2a). This explanation for differences in root growth efficiency is not sufficient, however, to account for all the species differences observed. A. rubrum, for example, showed high lateral root growth efficiency (Fig. 2b) but low growth rates up the nutrient gradient (Fig. 2c) at least partially due to low root growth rates (Fig. 2d).
Although our study was not designed to test hypotheses relating rooting habits to characteristics of the species’ natural habitats, the results do not support the idea that the root systems of plants from nutrient-rich sites are architecturally more plastic and more actively forage for nutrients than plants from infertile sites (Campbell and Grime 1989). For example, R. glabra, the species that expanded the fastest below-ground with the most architectural plasticity and highest efficiency is characteristic of infertile and rocky soil. Similarly, B. populifolia, the tree species with the most rapid root growth rates up the nutrient gradient (Fig. 2c), is common on rocky outcrops where it roots in small pockets of soil that collect in spatially isolated cracks and crevices. In a related study of soil resource heterogeneity in the study region, Kelly and Canham (1992) found the highest spatial variation in sites with the lowest average resource availability. These results suggests that root foraging efficiency is most beneficial for species that typically occur on poor and spatially heterogeneous soils. Thus, the apparent inefficiencies in root foraging of the shrubs V. dentatum and C. racemosa and the trees F. americana and A. rubrum may be related to their occurrence in more fertile and relatively homogeneous soils.
Complicating interpretation of the main finding of this study that shrubs and trees do not differ in nutrient foraging speeds or efficiencies is that the species that is best at both forages with a combination of rhizomes and roots. Those rhizomes are multifunctional insofar as they promote rapid and efficient colonization of resource-rich patches of soil while also allowing rapid clonal expansion. This capacity to spread vegetatively contributes to the invasiveness of a similar species of Rhus (R. typhina) where introduced in China (Wang et al. 2008). It is important to note that because we worked only with seedlings we missed any shrub-tree differences that may emerge when trees grow large stems and thick structural roots.
Conclusions
Despite our failure to find consistent differences in belowground exploration rates of the shrub and tree species used in this pot study, comparisons of the rates of soil patch discovery by plants of different growth forms could yield interesting results. To date, the voluminous literature on root foraging is dominated by studies of root foraging by herbaceous species, particularly cultivated cereals (Freschet et al. 2021; Lynch 2022; van der Heijden et al. 2015; White et al. 2013; de Kroon and Mommer 2006; Jing et al. 2022) but it would be interesting to extend these studies to woody species. By comparing congeneric shrubs and trees from the same native habitats, some limitations on the interpretation of results from our taxonomically uncontrolled study could be avoided but there are also advantages of experimenting with a wide variety of taxa. Future studies could also explore potential variation in root foraging ability related to the plant organ from which roots emerge such as from rhizomes, stolons (Zheng et al. 2022), or scrambling and fallen stems (Mori et al. 2021). Studies of arborescent monocots such as palms (Arecaceae) and pandans (Pandanaceae) could also be interesting insofar as they lack secondary growth and thus cannot produce thick roots (Tomlinson 1990). The results of such studies could inform the design of complex agroecosystems that include species with different growth forms (Lynch 2022; Jing et al. 2022). Finally, with advances in DNA-based root identification techniques (Jones et al. 2011), field experiments that avoid the many artifacts of pot studies are now feasible.
References
Campbell BD, Grime JP (1989) A comparative study of plant responsiveness to the duration of episodes of mineral nutrient enrichment. New Phytol 112:261–267. https://doi.org/10.1111/j.1469-8137.1989.tb02382.x
Campbell BD, Grime JP, Mackey JML (1991) A trade-off between scale and precision in resource foraging. Oecologia 87:532–538. https://doi.org/10.1007/BF00329417
de Kroon H, Mommer L (2006) Root foraging theory put to the test. Trends Ecol Evol 21:113–116. https://doi.org/10.1016/j.tree.2005.11.021
Fitzsimons JA, Michael DR (2017) Rocky outcrops: a hard road in the conservation of critical habitats. Biol Cons 211:36–44. https://doi.org/10.1016/j.biocon.2016.11.019
Freschet GT, Roumet C, Comas H, Weemstra M, Bengough AG, Rewald B, Bardgett RD, De Deyn GB, Johnson D, Klimešová J et al (2021) Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytol 232:1123–1158. https://doi.org/10.1111/nph.17072
Götmark F, Götmark E, Jensen AM (2016) Why be a shrub? A basic model and hypotheses for the adaptive values of a common growth form. Front Plant Sci 7:1095. https://doi.org/10.3389/fpls.2016.01095
Jing J, Gao W, Cheng L, Wang X, Duan F, Yuan L, Rengel Z, Zhang F, Li H, Cahill JF, Shen J (2022) Harnessing root-foraging capacity to improve nutrient-use efficiency for sustainable maize production. Field Crops Res 279:108462. https://doi.org/10.1016/j.fcr.2022.108462
Jones FA, Erickson DL, Bernal MA, Bermingham E, Kress WJ, Herre EA, Muller-Landau HC, Turner BL (2011) The roots of diversity: below ground species richness and rooting distributions in a tropical forest revealed by DNA barcodes and inverse modeling. PLoS ONE 6(9):e24506. https://doi.org/10.1371/journal.pone.0024506
Kelly VR, Canham CD (1992) Resource heterogeneity in oldfields. J Veg Sci 3:545–552. https://doi.org/10.2307/3235811
Larjavaara M (2015) Trees and shrubs differ biomechanically. Trends Ecol Evol 30:499–500. https://doi.org/10.1016/j.tree.2015.07.007
Lynch JP (2022) Harnessing root architecture to address global challenges. Plant J 109:415–431. https://doi.org/10.1111/tpj.15560
Montgomery RA, Reich PB, Palik BJ (2010) Untangling positive and negative biotic interactions: views from above and below ground in a forest ecosystem. Ecology 91:3641–3655. https://doi.org/10.1890/09-1663.1
Mori H, Ueno S, Kamijo T, Tsumura Y, Masaki T (2021) Interspecific variation in clonality in temperate lianas revealed by genetic analysis: do clonal proliferation processes differ among lianas? Plant Species Biol 36:578–588. https://doi.org/10.1111/1442-1984.12348
Ostertag R (1998) Belowground effects of canopy gaps in a tropical wet forest. Ecology 79:1294–1304. https://doi.org/10.1890/0012-9658(1998)079[1294:BEOCGI]2.0.CO;2
Putz FE (2023) Climbing plants beat trees to soil nutrient patches. Curr Biol 33:R659–R676. https://doi.org/10.1016/j.cub.2023.04.035
Putz FE, Canham CD (1992) Mechanisms of arrested succession in shrublands: root and shoot competition between shrubs and tree seedlings. For Ecol Manage 49:267–275. https://doi.org/10.1016/0378-1127(92)90140-5
Putz FE, Holbrook NM (1989) Strangler fig rooting habits and nutrient relations in the llanos of Venezuela. Amer J Bot 76:781–788. https://doi.org/10.1002/j.1537-2197.1989.tb15056.x
Sanford RL (1989) Fine root biomass under a tropical forest in light gap openings in Costa Rica. J Trop Ecol 5:251–256. https://doi.org/10.1017/s02664740003575
SAS Institute, Inc. (1987) SAS/STAT guide for personal computers, version, 6th edn. SAS Institute, Inc., Cary, North Carolina, USA
Tomlinson PB (1990) The structural biology of palms. Oxford University Press, Oxford, UK, p 447
van Der Heijden MG, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423. https://doi.org/10.1111/nph.13288
Wang G, Jiang G, Yu S, Li Y, Liu H (2008) Invasion possibility and potential effects of Rhus typhina on Beijing municipality. JIPB 50:522–530. https://doi.org/10.1111/j.1744-7909.2008.00660.x
Wen Z, White PJ, Shen J, Lambers H (2022) Linking root exudation to belowground economic traits for resource acquisition. New Phytol 233:1620–1635. https://doi.org/10.1111/nph.17854
White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM (2013) Matching roots to their environment. Ann Bot 112:207–222. https://doi.org/10.1093/aob/mct123
Wilczynski CJ, Pickett STA (1993) Fine root biomass within experimental canopy gaps: evidence for a below-ground gap. J Veg Sci 4:571–574. https://doi.org/10.2307/3236086
Zheng X, Gao Y, Wang Y, Xing F, Zhao M, Gao Y (2022) Optimal foraging strategies in varying nutrient heterogeneity: responses of a stoloniferous clonal plant to patch pattern, size and quality. Écoscience 29:221–232. https://doi.org/10.1080/11956860.2022.2048533
Acknowledgements
We thank Emma Macintosh, Rebecca Ostertag, Claudia Romero, Philip J. Burton, and two anonymous reviewers for comments on earlier versions of this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by the Central Hudson Gas and Electric Corporation, the Empire State Electric Energy Research Corporation, NSF Grants BSR-902003 and DEB-9220620, and the Mary Flagler Cary Charitable Trust.
Author information
Authors and Affiliations
Contributions
FEP and CDC conceptualized the study, SVO assisted with data collection, CDC analyzed the data, FEP wrote the first draft that was revised by CDC and SVO.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Data availability
All data will be made available by the Environmental Data Initiative (EDI) after acceptance.
Additional information
Communicated by Claus Holzapfel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Putz, F.E., Canham, C.D. & Ollinger, S.V. Belowground exploration by trees and shrubs. Plant Ecol 225, 605–610 (2024). https://doi.org/10.1007/s11258-024-01416-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-024-01416-7