Abstract
Background
Diabetic nephropathy (DN) is a severe microvascular complication of diabetes mellitus and a primary reason for end-stage renal disease (ESRD). Isorhapontigenin (ISO), a natural derivative of stilbene, has significant anti-inflammatory and antioxidant effects. Nevertheless, its impact on DN remains elusive.
Methods
Human vascular endothelial cells (HUVECs) and podocytes were damaged by high glucose (HG). Cell viability and apoptosis were testified by the cell counting kit-8 (CCK-8) assay and flow cytometry, respectively. The mRNA profiles of antioxidant factors HO-1, NQO1, and Prx1 were monitored by real-time quantitative polymerase chain reaction (RT-qPCR). Western blotting (WB) was implemented to verify the expression of apoptosis-related proteins (Bax, Bad, and Bcl-XL), antioxidant factors (HO-1, NQO1, and Prx1), autophagy-related proteins (Beclin-1, ATG5, p62), podocalyxin (podocin, nephrin, and synaptopodin) and the AMPK/Nrf2 pathway. The levels of oxidative stress-related markers MDA, SOD and CAT were assessed with the corresponding kits. Compound C (CC), an inhibitor of AMPK, was deployed to probe the effects of modulating the AMPK/Nrf2 pathway on ISO in oxidative stress and autophagy in HUVECs and podocytes. Streptozotocin (STZ) was injected intraperitoneally into mice to establish an animal model of diabetes mellitus and to clarify the impact of ISO on the renal parameters such as serum creatinine, urea nitrogen and urinary protein in diabetic mice.
Results
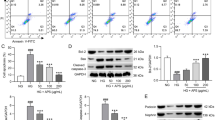
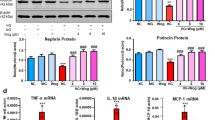
ISO notably facilitated cell proliferation, impeded apoptosis, elevated the expression of antioxidant-related factors, alleviated HG-induced oxidative stress and activated autophagy in HUVECs and podocytes. ISO activated the AMPK/Nrf2 pathway. Attenuating AMPK diminished the protective effect of ISO on HUVECs and podocytes, curbed cell proliferation, intensified apoptosis and oxidative stress, and dampened autophagy. In-vivo experiments also displayed that ISO reduced histopathological damage, lowered serum creatinine, urea nitrogen and urinary ACR levels, and eased kidney damage in DN mice.
Conclusion
ISO attenuates HG-induced oxidative stress and activates autophagy by motivating the AMPK/Nrf2 pathway, exerting a protective effect on HUVECs and podocytes and reducing renal injury in DN mice.







Similar content being viewed by others
Data availability
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014 Jan;63(1 Suppl):A7. https://doi.org/10.1053/j.ajkd.2013.11.001. PMID: 24360288.
Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, Fort PE. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019 Sep;62(9):1539–1549. https://doi.org/10.1007/s00125-019-4959-1. Epub 2019 Jul 25. PMID: 31346658; PMCID: PMC6679814.
American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020 Jan;43(Suppl 1):S135-S151. https://doi.org/10.2337/dc20-S011. PMID: 31862754.
Drummond KN, Kramer MS, Suissa S, Lévy-Marchal C, Dell'Aniello S, Sinaiko A, Mauer M; International Diabetic Nephropathy Study Group. Effects of duration and age at onset of type 1 diabetes on preclinical manifestations of nephropathy. Diabetes. 2003 Jul;52(7):1818–24. https://doi.org/10.2337/diabetes.52.7.1818. PMID: 12829652.
Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014 Jun;124(6):2333–40. https://doi.org/10.1172/JCI72271. Epub 2014 Jun 2. PMID: 24892707; PMCID: PMC4089448.
Meyer TW, Bennett PH, Nelson RG (1999 Nov) Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42(11):1341–1344. https://doi.org/10.1007/s001250051447 (PMID: 10550418)
Kaverina NV, Eng DG, Schneider RR, Pippin JW, Shankland SJ (2016) Partial podocyte replenishment in experimental FSGS derives from nonpodocyte sources. Am J Physiol Renal Physiol 310(11):F1397–F1413. https://doi.org/10.1152/ajprenal.00369.2015 (Epub 2016 Apr 13 PMID: 27076646)
Kuwabara A, Satoh M, Tomita N, Sasaki T, Kashihara N (2010) Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia 53(9):2056–2065. https://doi.org/10.1007/s00125-010-1810-0 (Epub 2010 Jun 6 PMID: 20526760)
Tabit CE, Chung WB, Hamburg NM, Vita JA (2010) Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord 11(1):61–74. https://doi.org/10.1007/s11154-010-9134-4 (PMID: 20186491)
Dounousi E, Duni A, Leivaditis K, Vaios V, Eleftheriadis T, Liakopoulos V. Improvements in the Management of Diabetic Nephropathy. Rev Diabet Stud. 2015 Spring-Summer;12(1–2):119–33. doi: https://doi.org/10.1900/RDS.2015.12.119. Epub 2015 Aug 10. PMID: 26676665.
Wang QL, Lin M, Liu GT (2001) anti-oxidative activity of natural isorhapontigenin. Jpn J Pharmacol 87(1):61–66. https://doi.org/10.1254/jjp.87.61 (PMID: 11676199)
Gu Y, Wang T, Chen J, Zhou Z, Wang Y, Chen J, Liu N, Jiang Z (2020) The Chinese Herb Codonopsis pilosula Isolate Isorhapontigenin protects against oxidative stress injury by inhibiting the activation of PI3K/Akt signaling pathway. J Integr Neurosci 19(2):333–340. https://doi.org/10.31083/j.jin.2020.02.1152 (PMID: 32706197)
Ma Y, Tu C, Liu W, Xiao Y, Wu H (2019) Isorhapontigenin suppresses interleukin-1β-induced inflammation and cartilage matrix damage in rat chondrocytes. Inflammation 42(6):2278–2285. https://doi.org/10.1007/s10753-019-01092-0 (PMID: 31512108)
Chu XY, Yang SZ, Zhu MQ, Zhang DY, Shi XC, Xia B,.et al. Isorhapontigenin Improves Diabetes in Mice via Regulating the Activity and Stability of PPARγ in Adipocytes. J Agric Food Chem. 2020 Apr 1;68(13):3976–3985. doi: https://doi.org/10.1021/acs.jafc.0c00515. Epub 2020 Mar 23. PMID: 32178518.
Steinberg GR, Schertzer JD (2014) AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: implications for diabetes and cardiovascular disease. Immunol Cell Biol 92(4):340–345. https://doi.org/10.1038/icb.2014.11 (Epub 2014 Mar 18 PMID: 24638063)
da Costa RM, Rodrigues D, Pereira CA, Silva JF, Alves JV, Lobato NS, Tostes RC (2019) Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front Pharmacol 12(10):382. https://doi.org/10.3389/fphar.2019.00382.PMID:31031630;PMCID:PMC6473049
Shen Y, Liu X, Shi J, Wu X (2019) Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol 15(125):496–502. https://doi.org/10.1016/j.ijbiomac.2018.11.190 (Epub 2018 Nov 20 PMID: 30468811)
Zhou F, Wang M, Ju J, Wang Y, Liu Z, Zhao X et al. Schizandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am J Transl Res. 2019 Jan 15;11(1):199–209. PMID: 30787979; PMCID: PMC6357305.
Ma T, Zheng Z, Guo H, Lian X, Rane MJ, Cai L et al. 4-O-methylhonokiol ameliorates type 2 diabetes-induced nephropathy in mice likely by activation of AMPK-mediated fatty acid oxidation and Nrf2-mediated anti-oxidative stress. Toxicol Appl Pharmacol. 2019 May 1;370:93–105. https://doi.org/10.1016/j.taap.2019.03.007. (Epub 2019 Mar 12. PMID: 30876865).
Kim BH, Lee ES, Choi R, Nawaboot J, Lee MY, Lee EY,.et al. Protective effects of curcumin on renal oxidative stress and lipid metabolism in a rat model of type 2 diabetic nephropathy. Yonsei Med J. 2016 May;57(3):664–73. https://doi.org/10.3349/ymj.2016.57.3.664. (PMID: 26996567; PMCID: PMC4800357).
Yang WY, Chen LC, Jhuang YT, Lin YJ, Hung PY, Ko YC et al. Injection of hybrid 3D spheroids composed of podocytes, mesenchymal stem cells, and vascular endothelial cells into the renal cortex improves kidney function and replenishes glomerular podocytes. Bioeng Transl Med. 2021 Jan 21;6(2):e10212. https://doi.org/10.1002/btm2.10212. (PMID: 34027096).
Dasgupta B, Seibel W (2018) Compound C/Dorsomorphin: its use and misuse as an AMPK inhibitor. Methods Mol Biol 1732:195–202. https://doi.org/10.1007/978-1-4939-7598-3_12 (PMID: 29480476)
Stieger N, Worthmann K, Teng B, Engeli S, Das AM, Haller H, Schiffer M (2012) Impact of high glucose and transforming growth factor-β on bioenergetic profiles in podocytes. Metabolism 61(8):1073–1086. https://doi.org/10.1016/j.metabol.2011.12.003 (Epub 2012 Feb 24 PMID: 22365040)
Lin JS, Susztak K (2016) Podocytes: The weakest link in diabetic kidney disease? Curr Diab Rep 16(5):45. https://doi.org/10.1007/s11892-016-0735-5 (PMID: 27053072)
Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N,.et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014 Apr;124(4):1608–21. https://doi.org/10.1172/JCI71195. ( Epub 2014 Mar 3. PMID: 24590287).
Ebefors K, Wiener RJ, Yu L, Azeloglu EU, Yi Z, Jia F et al. Endothelin receptor-A mediates degradation of the glomerular endothelial surface layer via pathologic crosstalk between activated podocytes and glomerular endothelial cells. Kidney Int. 2019 Oct;96(4):957–970. https://doi.org/10.1016/j.kint.2019.05.007. (Epub 2019 May 22. PMID: 31402170).
Qi H, Casalena G, Shi S, Yu L, Ebefors K, Sun Y et al. Glomerular Endothelial Mitochondrial Dysfunction Is Essential and Characteristic of Diabetic Kidney Disease Susceptibility. Diabetes. 2017 Mar;66(3):763–778. https://doi.org/10.2337/db16-0695. (Epub 2016 Nov 29. PMID: 27899487).
Zheng X, Soroush F, Long J, Hall ET, Adishesha PK, Bhattacharya S,.et al. Murine glomerular transcriptome links endothelial cell-specific molecule-1 deficiency with susceptibility to diabetic nephropathy. PLoS One. 2017 Sep 21;12(9):e0185250. https://doi.org/10.1371/journal.pone.0185250. (PMID: 28934365).
Pecyna P, Wargula J, Murias M, Kucinska M (2020) More than resveratrol: new insights into stilbene-based compounds. Biomolecules 10(8):1111. https://doi.org/10.3390/biom10081111 (PMID: 32726968)
Sun X, Cui X (2020) Isorhapontigenin alleviates cerebral ischemia/reperfusion injuries in rats and modulated the PI3K/Akt signaling pathway. Naunyn Schmiedebergs Arch Pharmacol 393(9):1753–1760. https://doi.org/10.1007/s00210-019-01794-0 (Epub 2020 Jan 4 PMID: 31900521)
Wang P, Wang M, Hu Y, Chen J, Cao Y, Liu C et al. Isorhapontigenin protects against doxorubicin-induced cardiotoxicity via increasing YAP1 expression. Acta Pharm Sin B. 2021 Mar;11(3):680–693. https://doi.org/10.1016/j.apsb.2020.10.017. (Epub 2020 Nov 1. PMID: 33777675; PMCID: PMC7982427).
Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR (2004) Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem 52(15):4713–4719. https://doi.org/10.1021/jf040095e (PMID: 15264904)
Zhang Y, Ren S, Ji Y, Liang Y (2019) Pterostilbene ameliorates nephropathy injury in streptozotocin-induced diabetic rats. Pharmacology 104(1–2):71–80. https://doi.org/10.1159/000500293 (Epub 2019 May 22 PMID: 31117104)
Wen D, Huang X, Zhang M, Zhang L, Chen J, Gu Y, Hao CM (2013) Resveratrol attenuates diabetic nephropathy via modulating angiogenesis. PLoS ONE 8(12):e82336. https://doi.org/10.1371/journal.pone.0082336 (PMID: 24312656)
Borgohain MP, Lahkar M, Ahmed S, Chowdhury L, Kumar S, Pant R, Choubey A (2017) Small molecule inhibiting nuclear factor-kB ameliorates oxidative stress and suppresses renal inflammation in early stage of alloxan-induced diabetic nephropathy in rat. Basic Clin Pharmacol Toxicol 120(5):442–449. https://doi.org/10.1111/bcpt.12718 (Epub 2017 Jan 23 PMID: 27888584)
Koch EAT, Nakhoul R, Nakhoul F, Nakhoul N (2020) Autophagy in diabetic nephropathy: a review. Int Urol Nephrol 52(9):1705–1712. https://doi.org/10.1007/s11255-020-02545-4 (Epub 2020 Jul 13 PMID: 32661628)
Casalena GA, Yu L, Gil R, Rodriguez S, Sosa S, Janssen W, Azeloglu EU, Leventhal JS, Daehn IS (2020) The diabetic microenvironment causes mitochondrial oxidative stress in glomerular endothelial cells and pathological crosstalk with podocytes. Cell Commun Signal 18(1):105. https://doi.org/10.1186/s12964-020-00605-x (PMID: 32641054)
Wang W, Ding XQ, Gu TT, Song L, Li JM, Xue QC, Kong LD (2015) Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377. Free Radic Biol Med 83:214–226. https://doi.org/10.1016/j.freeradbiomed.2015.02.029 (Epub 2015 Mar 5 PMID: 25746774)
Xu XH, Ding DF, Yong HJ, Dong CL, You N, Ye XL,.et al. Resveratrol transcriptionally regulates miRNA-18a-5p expression ameliorating diabetic nephropathy via increasing autophagy. Eur Rev Med Pharmacol Sci. 2017 Nov;21(21):4952–4965. (PMID: 29164562).
Li Z, Guo H, Li J, Ma T, Zhou S, Zhang Z, Miao L, Cai L (2020) Sulforaphane prevents type 2 diabetes-induced nephropathy via AMPK-mediated activation of lipid metabolic pathways and Nrf2 anti-oxidative function. Clin Sci (Lond) 134(18):2469–2487. https://doi.org/10.1042/CS20191088 (PMID: 32940670)
Cheng Y, Zhang X, Ma F, Sun W, Wang W, Yu J, Shi Y, Cai L, Xu Z (2020) The role of Akt2 in the protective effect of fenofibrate against diabetic nephropathy. Int J Biol Sci 16(4):553–567. https://doi.org/10.7150/ijbs.40643.PMID:32025205;PMCID:PMC6990917
Xie R, Zhang H, Wang XZ, Yang XZ, Wu SN, Wang HG, Shen P, Ma TH (2017) The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats. Food Funct 8(1):299–306. https://doi.org/10.1039/c6fo01601d (PMID: 28009869)
Wada J, Makino H (2013) Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 124(3):139–152. https://doi.org/10.1042/CS20120198 (PMID: 23075333)
Khoshjou F, Dadras F (2014) Mitochondrion and its role in diabetic nephropathy. Iran J Kidney Dis 8(5):355–358 (PMID: 25194400)
Dadras F, Khoshjou F (2015) Endoplasmic reticulum and its role in diabetic nephropathy. Iran J Kidney Dis 9(4):267–272 (PMID: 26174452)
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not- for- profit sectors.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: HT; Performed the experiments: HT, XZ; Statistical analysis: XZ, HW; Wrote the paper: HT, XZ, HW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics statement
Our study was approved by the Animal Research Ethics Committee of Beijing Daxing District People’s Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, H., Zheng, X. & Wang, H. Isorhapontigenin ameliorates high glucose-induced podocyte and vascular endothelial cell injuries via mitigating oxidative stress and autophagy through the AMPK/Nrf2 pathway. Int Urol Nephrol 55, 423–436 (2023). https://doi.org/10.1007/s11255-022-03325-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03325-y