Abstract
Background
Specificity protein 1 (Sp1) is a transcription factor that exerts key functions in the carcinogenesis and progression of various types of cancer. However, its expression and prognostic value in bladder urothelial carcinoma (BUC) have yet to be completely elucidated.
Methods
The present study performed reverse transcription-quantitative polymerase chain reaction (RT-qPCR) to examine Sp1 mRNA expression in 12 pairs of urothelial carcinoma and adjacent normal bladder tissues. Immunohistochemistry (IHC) was performed in 113 paraffin-embedded urothelial carcinoma tissues to detect the expression of Sp1. Kaplan–Meier plots and Cox proportional hazards regression model were used to analyze the correlation between Sp1 expression and patient prognosis.
Results
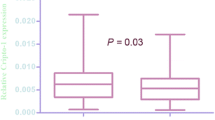
The mRNA expression of Sp1 was elevated in the urothelial carcinoma by RT-qPCR compared with their paired normal bladder tissues. Among 113 cases of patients with urothelial carcinoma, there were 39 low histological grade and 74 high histological grade, 61 unifocal tumor and 52 multifocal tumor, 78 cases in Ta, T1, and T2 stages, and 35 cases in T3 and T4 stages. The enhanced expression of Sp1 mRNA was observed in tumors with a high histological grade, and invasive and metastatic samples. Immunohistochemistry revealed that Sp1 high expression was significantly correlated with the histological grade, tumor stage, vascular invasion, lymph node metastasis and distant metastasis (P < 0.05). Kaplan–Meier analysis demonstrated that elevated Sp1 expression in cancer tissue was correlated with a significantly poor overall survival (OS) and disease-free survival (DFS) compared with samples with low Sp1 expression (P < 0.05). Multivariate analyses by Cox’s proportional hazard model also revealed that the expression of Sp1 was an independent prognostic factor in urothelial carcinoma.
Conclusion
Sp1 expression is significantly elevated in urothelial carcinoma and may be used to identify a subset of patients with aggressive behaviors and poor clinical outcomes. Sp1 is a potential novel independent prognostic biomarker for patients with urothelial carcinoma following surgery.



Similar content being viewed by others
References
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132. https://doi.org/10.3322/caac.21338
Smaldone MC, Jacobs BL, Smaldone AM, Hrebinko RL Jr (2008) Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology 72:613–616. https://doi.org/10.1016/j.urology.2008.04.052
Cookson MS (2005) The surgical management of muscle invasive bladder cancer: a contemporary review. Semin Radiat Oncol 15:10–18. https://doi.org/10.1016/j.semradonc.2004.07.009
Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG (2001) Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 19:666–675. https://doi.org/10.1200/JCO.2001.19.3.666
Safe S, Imanirad P, Sreevalsan S, Nair V, Jutooru I (2014) Transcription factor Sp1, also known as specificity protein 1 as a therapeutic target. Expert Opin Ther Targets 18:759–769. https://doi.org/10.1517/14728222.2014.914173
Zhao Y, Zhang W, Guo Z, Ma F, Wu Y, Bai Y, Gong W, Chen Y, Cheng T, Zhi F, Zhang Y, Wang J, Jiang B (2013) Inhibition of the transcription factor Sp1 suppresses colon cancer stem cell growth and induces apoptosis in vitro and in nude mouse xenografts. Oncol Rep 30:1782–1792. https://doi.org/10.3892/or.2013.2627
Hirose T, Horvitz HR (2013) An Sp1 transcription factor coordinates caspase-dependent and -independent apoptotic pathways. Nature 500:354–358. https://doi.org/10.1038/nature12329
Wang YT, Yang WB, Chang WC, Hung JJ (2011) Interplay of posttranslational modifications in Sp1 mediates Sp1 stability during cell cycle progression. J Mol Biol 414:1–14. https://doi.org/10.1016/j.jmb.2011.09.027
Zhu J, Sun Y, Luo J, Wu M, Li J, Cao Y (2015) Specificity protein 1 regulates gene expression related to fatty acid metabolism in goat mammary epithelial cells. Int J Mol Sci 16(1):1806–1820. https://doi.org/10.3390/ijms16011806
Chen Y, Huang Y, Huang Y, Xia X, Zhang J, Zhou Y, Tan Y, He S, Qiang F, Li A, Re OD, Li G, Zhou J (2014) JWA suppresses tumor angiogenesis via Sp1-activated matrix metalloproteinase-2 and its prognostic significance in human gastric cancer. Carcinogenesis 35:442–451. https://doi.org/10.1093/carcin/bgt311
Beishline K (2015) Azizkhan-Clifford J (2015) Sp1 and the “hallmarks of cancer.” FEBS J 282(2):224–258. https://doi.org/10.1111/febs.13148
Jiang NY, Woda BA, Banner BF, Whalen GF, Dresser KA, Lu D (2008) Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev 17:1648–1652. https://doi.org/10.1158/1055-9965.EPI-07-2791
Jiang W, Jin Z, Zhou F, Cui J, Wang L, Wang L (2015) High co-expression of Sp1 and HER-2 is correlated with poor prognosis of gastric cancer patients. Surg Oncol 24:220–225. https://doi.org/10.1016/j.suronc.2015.05.004
Guan H, Cai J, Zhang N, Wu J, Yuan J, Li J, Li M (2012) Sp1 is upregulated in human glioma, promotes MMP-2-mediated cell invasion and predicts poor clinical outcome. Int J Cancer 130:593–601. https://doi.org/10.1002/ijc.26049
Wang J, Kang M, Qin YT, Wei ZX, Xiao JJ, Wang RS (2015) Sp1 is over-expressed in nasopharyngeal cancer and is a poor prognostic indicator for patients receiving radiotherapy. Int J Clin Exp Pathol 8:6936–6943
Greene FL, Page DL, Fleming ID, April F, Balch CM, Haller DG, Monica M (2002) American Joint Committee on Cancer (AJCC) staging manual, 6th edn. Springer, Philadelphia
Livak KJ (2001) Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Li HT, Duymich CE, Weisenberger DJ, Liang G (2016) Genetic and epigenetic alterations in bladder cancer. Int Neurourol J 20:S84–S94. https://doi.org/10.5213/inj.1632752.376
Abbosh PH, McConkey DJ, Plimack ER (2015) Targeting signaling transduction pathways in bladder cancer. Curr Oncol Rep 17:58. https://doi.org/10.1007/s11912-015-0477-6
Song BN, Kim SK, Chu IS (2017) Bioinformatic identification of prognostic signature defined by copy number alteration and expression of CCNE1 in non-muscle invasivebladder cancer. Exp Mol Med 49:e282. https://doi.org/10.1038/emm.2016.120
Devanand P, Kim SI, Choi YW, Sheen SS, Yim H, Ryu MS, Kim SJ, Kim WJ, Lim IK (2014) Inhibition of bladder cancer invasion by Sp1-mediated BTG2 expression via inhibition of DNA methyltransferase 1. FEBS J 281:5581–5601. https://doi.org/10.1111/febs.13099
Zhou C, Ji J, Cai Q, Shi M, Chen X, Yu Y, Liu B, Zhu Z, Zhang J (2013) MTA2 promotes gastric cancer cells invasion and is transcriptionally regulated by Sp1. Mol Cancer 12:102. https://doi.org/10.1186/1476-4598-12-102
Nam EH, Lee Y, Zhao XF, Park YK, Lee JW, Kim S (2014) ZEB2-Sp1 cooperation induces invasion by upregulating cadherin-11 and integrin α5 expression. Carcinogenesis 35:302–314. https://doi.org/10.1093/carcin/bgt340
Wang Q, Qian J, Wang F, Ma Z (2012) Cellular prion protein accelerates colorectal cancer metastasis via the Fyn-SP1-SATB1 axis. Oncol Rep 28:2029–2034. https://doi.org/10.3892/or.2012.2025
Zhao J, Ye W, Wu J, Liu L, Yang L, Gao L, Chen B, Zhang F, Yang H, Li Y (2015) Sp1-CD147 positive feedback loop promotes the invasion ability of ovarian cancer. Oncol Rep 34:67–76. https://doi.org/10.3892/or.2015.3999
Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R 3rd, Li X, Safe S (2008) Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res 68:5345–5354. https://doi.org/10.1158/0008-5472.CAN-07-6805
Chadalapaka G, Jutooru I, Burghardt R, Safe S (2010) Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol Cancer Res 8:739–750. https://doi.org/10.1158/1541-7786.MCR-09-0493
Jutooru I, Chadalapaka G, Sreevalsan S, Lei P, Barhoumi R, Burghardt R, Safe S (2010) Arsenic trioxide downregulates specificity protein (Sp) transcription factors and inhibits bladder cancer cell and tumor growth. Exp Cell Res 316:2174–2188. https://doi.org/10.1016/j.yexcr.2010.04.027
Chadalapaka G, Jutooru I, Safe S (2012) Celastrol decreases specificity proteins (Sp) and fibroblast growth factor receptor-3 (FGFR3) in bladder cancer cells. Carcinogenesis 33:886–894. https://doi.org/10.1093/carcin/bgs102
Bedolla RG, Gong J, Prihoda TJ, Yeh IT, Thompson IM, Ghosh R, Kumar AP (2012) Predictive value of Sp1/Sp3/FLIP signature for prostate cancer recurrence. PLoS ONE 7:e44917. https://doi.org/10.1371/journal.pone.0044917
Hu J, Hu H, Hang JJ, Yang HY, Wang ZY, Wang L, Chen DH, Wang LW (2016) Simultaneous high expression of PLD1 and Sp1 predicts a poor prognosis for pancreatic ductal adenocarcinoma patients. Oncotarget 7:78557–78565. https://doi.org/10.18632/oncotarget.12447
Author information
Authors and Affiliations
Contributions
JZ analyzed the data, wrote the manuscript and performed the experiment of immunohistochemistry; MK performed the experiment and analyzed the data; ZL analyzed the data; CG designed the study and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, J., Lu, Z., Ke, M. et al. Sp1 is overexpressed and associated with progression and poor prognosis in bladder urothelial carcinoma patients. Int Urol Nephrol 54, 1505–1512 (2022). https://doi.org/10.1007/s11255-022-03212-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03212-6