Abstract
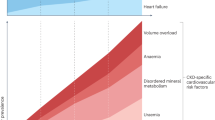
The Klotho gene displays an extremely shortened life span with loss of function missense mutations leading to premature multiple organ failure, thus resembling human premature aging syndromes. The transmembrane form of Klotho protein functions as an obligatory co-receptor for FGF23. Klotho and FGF23 are crucial components for the regulation of vitamin D metabolism and subsequently blood phosphate levels. The secreted Klotho protein has multiple regulatory functions, including effects on electrolyte homeostasis, on growth factor pathways as well as on oxidative stress, which are currently the object of extensive research. Klotho protein deficiency is observed in many experimental and clinical disease models. Genetic polymorphisms such as the G-395A polymorphism in the promoter region of the Klotho gene have been associated with the development of essential hypertension. The kidneys are the primary site of Klotho production, and renal Klotho is decreased in CKD, followed by a reduction in plasma Klotho. Klotho deficiency has been both associated with progression of CKD as well as with its cardinal systemic manifestations, including cardiovascular disease. Thus, Klotho has been suggested both as a risk biomarker for early detection of CKD and additionally as a potential therapeutic tool in the future.



Similar content being viewed by others
References
Kuro-o M, Matsumura Y, Aizawa H et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Shiraki-Iida T, Aizawa H, Matsumura Y et al (1998) Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424:6–10
Hu MC, Shi M, Zhang J et al (2010) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24:3438–3450
Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y (1988) Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 26:626–630
Hu MC, Kuro-o M, Moe OW (2013) Renal and extrarenal actions of Klotho. Semin Nephrol 33:118–129
Kuro-o M (2006) Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens 15:437–441
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP et al (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123
Kuro-o M (2009) Klotho and aging. Biochim Biophys Acta 1790:1049–1058
Moe OW (2009) PiT-2 coming out of the pits. Am J Physiol Renal Physiol 296:F689–F690
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310(5747):490–493
Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro O, Huang CL (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105(28):9805–9810
Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL (2009) Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol 76:38–46
Tosato M, Zamboni V, Ferrini A, Cesari M (2007) The aging process and potential interventions to extend life expectancy. Clin Interv Aging 2:401–412
Sugiura H, Tsuchiya K, Nitta K (2011) Circulating levels of soluble alpha-Klotho in patients with chronic kidney disease. Clin Exp Nephrol 15:795–796
Stenvinkel P, Larsson TE (2013) Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis 62:339–351
Yamamoto M, Clark JD, Pastor JV et al (2005) Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 11(280):38029–38034
Maekawa Y, Ishikawa K, Yasuda O et al (2009) Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine 35:341–346
Maekawa Y, Ohishi M, Ikushima M et al (2011) Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int 11:510–516
Sugiura H, Yoshida T, Shiohira S et al (2012) Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol 15(302):F1252–F1264
Hu MC, Shi M, Zhang J et al (2011) Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22(1):124–136
Olauson H, Lindberg K, Amin R et al (2012) Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol 23:1641–1651
Sitara D, Kim S, Razzaque MS et al (2008) Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet 4(8):e1000154
Ohnishi M, Nakatani T, Lanske B, Razzaque MS (2009) Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int 75:1166–1172
Ohnishi M, Razzaque MS (2010) Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J 24:3562–3571
Block GA, Hulbert-Shearon TE, Levin NW, Port FK (1998) Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31:607–617
Dhingra R, Sullivan LM, Fox CS et al (2007) Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 14(167):879–885
Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA (2009) Serum phosphate and left ventricular hypertrophy in young adults: the coronary artery risk development in young adults study. Kidney Blood Press Res 32:37–44
Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA (2008) The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol 19:1092–1105
Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G (2005) Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 25(112):2627–2633
Tatar M, Bartke A, Antebi A (2003) The endocrine regulation of aging by insulin-like signals. Science 299(5611):1346–1351
Utsugi T, Ohno T, Ohyama Y et al (2000) Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism 49:1118–1123
Kurosu H, Yamamoto M, Clark JD et al (2005) Suppression of aging in mice by the hormone Klotho. Science 309(5742):1829–1833
Wolf I, Levanon-Cohen S, Bose S et al (2008) Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene 27(56):7094–7105
Liu H, Fergusson MM, Castilho RM et al (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317(5839):803–806
Mancia G, Fagard R, Narkiewicz K et al (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31:1281–1357
Osanai T, Kanazawa T, Yokono Y, Uemura T, Okuguchi T, Onodera K (1993) Effect of aging on sensitivity of blood pressure to salt. Nihon Ronen Igakkai Zasshi 30:30–34
Weinberger MH, Fineberg NS (1991) Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension 18:67–71
Xiao NM, Zhang YM, Zheng Q, Gu J (2004) Klotho is a serum factor related to human aging. Chin Med J (Engl) 117:742–747
Zhou X, Chen K, Lei H, Sun Z (2015) Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26:121–132
Zhou L, Mo H, Miao J et al (2015) Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol 185:3211–3223
Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L (2013) Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol 8:1899–1905
Lin Y, Chen J, Sun Z (2016) Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension 67:564–573
Andrukhova O, Slavic S, Smorodchenko A et al (2014) FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 6:744–759
Arking DE, Krebsova A, Macek M et al (2002) Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA 22(99):856–861
Imamura A, Okumura K, Ogawa Y et al (2006) Klotho gene polymorphism may be a genetic risk factor for atherosclerotic coronary artery disease but not for vasospastic angina in Japanese. Clin Chim Acta 371:66–70
Kim Y, Kim JH, Nam YJ et al (2006) Klotho is a genetic risk factor for ischemic stroke caused by cardioembolism in Korean females. Neurosci Lett 30(407):189–194
Rhee EJ, Oh KW, Yun EJ et al (2006) Relationship between polymorphisms G395A in promoter and C1818T in exon 4 of the KLOTHO gene with glucose metabolism and cardiovascular risk factors in Korean women. J Endocrinol Invest 29:613–618
Rhee EJ, Oh KW, Lee WY et al (2006) The differential effects of age on the association of KLOTHO gene polymorphisms with coronary artery disease. Metabolism 55:1344–1351
Shimoyama Y, Nishio K, Hamajima N, Niwa T (2009) KLOTHO gene polymorphisms G-395A and C1818T are associated with lipid and glucose metabolism, bone mineral density and systolic blood pressure in Japanese healthy subjects. Clin Chim Acta 406:134–138
Wang HL, Xu Q, Wang Z et al (2010) A potential regulatory single nucleotide polymorphism in the promoter of the Klotho gene may be associated with essential hypertension in the Chinese Han population. Clin Chim Acta 411:386–390
Wang Y, Sun Z (2009) Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54:810–817
Dammanahalli KJ, Sun Z (2008) Endothelins and NADPH oxidases in the cardiovascular system. Clin Exp Pharmacol Physiol 35(1):2–6
Sagar S, Kallo IJ, Kaul N, Ganguly NK, Sharma BK (1992) Oxygen free radicals in essential hypertension. Mol Cell Biochem 111:103–108
Saito Y, Nakamura T, Ohyama Y et al (2000) In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun 24(276):767–772
Bengtsson SH, Gulluyan LM, Dusting GJ, Drummond GR (2003) Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin Exp Pharmacol Physiol 30:849–854
Mazighi M, Pelle A, Gonzalez W et al (2004) IL-10 inhibits vascular smooth muscle cell activation in vitro and in vivo. Am J Physiol Heart Circ Physiol 287:H866–H871
Sugiura H, Yoshida T, Mitobe M et al (2010) Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant 25:60–68
Dai S, Zou Y, Togao O et al (2011) Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286:8655–8665
Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A (2003) Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112:1486–1494
Yang K, Wang C, Nie L et al (2015) Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol 26:2434–2446
Guan X, Nie L, He T et al (2014) Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. J Pathol 234:560–572
Zhao Y, Banerjee S, Dey N et al (2011) Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine) 536 phosphorylation. Diabetes 60:1907–1916
Liu F, Wu S, Ren H, Gu J (2011) Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 13:254–262
Xie J, Yoon J, An SW, Kuro-o M, Huang CL (2015) Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26:1150–1160
Sun CY, Chang SC, Wu MS (2012) Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int 81:640–650
Moreno JA, Izquierdo MC, Sanchez-Nino MD et al (2011) The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol 22:1315–1325
Hu MC, Shi M, Zhang J et al (2016) Renal production, uptake, and handling of circulating alphaklotho. J Am Soc Nephrol 27:79–90
Kadoya H, Satoh M, Haruna Y, Sasaki T, Kashihara N (2015) Klotho attenuates renal hypertrophy and glomerular injury in Ins2Akita diabetic mice. Clin Exp Nephrol. doi:10.1007/s10157-015-1202-3
Wang Y, Sun Z (2014) Antiaging gene Klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rats. J Hypertens 32:1629–1636
Kim AJ, Ro H, Kim H et al (2016) Klotho and S100A8/A9 as discriminative markers between pre-renal and intrinsic acute kidney injury. PLoS ONE 11(1):e0147255
Semba RD, Cappola AR, Sun K et al (2011) Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc 59:1596–1601
Bernheim J, Benchetrit S (2011) The potential roles of FGF23 and Klotho in the prognosis of renal and cardiovascular diseases. Nephrol Dial Transplant 26:2433–2438
Gao LL, Ding X, Xie DM, Yang M, Dong BR (2015) G-395A polymorphism in the promoter region of the KLOTHO gene and hypertension among elderly (90 years and older) Chinese individuals. Genet Mol Res 14:15444–15452
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kalaitzidis, R.G., Duni, A. & Siamopoulos, K.C. Klotho, the Holy Grail of the kidney: from salt sensitivity to chronic kidney disease. Int Urol Nephrol 48, 1657–1666 (2016). https://doi.org/10.1007/s11255-016-1325-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1325-9