Abstract
Bovine viral diarrhoea virus (BVDV) is a serious veterinary health concern worldwide. We conducted this study to determine the prevalence of persistent infections (PI) and identify the current strain among some dairy cattle herds in Egypt. A total of 240 serum samples were collected from six Egyptian provinces. Between 2019 and 2020, samples were tested by Enzyme linked immunosorbent assay (ELISA) for detection of PI animals, and then molecular characterization was performed. Six calves were found PI with a prevalence of 2.5% (6/240). Using molecular characterization, HoBi-like Pestivirus (BVD-3) was successfully identified in Egypt for the first time. Based on the BVD-3 reference strains on Genbank, the detected strains had an identity ranging from 98.8 to 99.6%. Partial nucleotide sequence of the 5′UTR gene for six tested samples was submitted to Genbank with accessions: OM324396, OM324397, OM324398, OM324399, OM3243100, and OM3243101.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that (BVDV) causes endemic disease in cattle, which has a profound impact on the worldwide economy because of immunosuppression, ill-thrift, premature culling, and a potentially fatal hemorrhagic syndrome called mucosal disease (Khodakaram-Tafti and Farjanikish 2017). BVDV is present in most cattle-producing countries, and according to Richter et al. (2019), since 1960, 107 countries have reported implementing mitigation activities.
Even though BVDV took its name from the bovine first host, the virus still infects approximately forty species, and most wild ruminants are susceptible to BVD virus infection, according to serological evidence. Besides wildlife, various non-bovid species are also thought to carry and spread the disease, although there is evidence of transient infection (TI) within most animals, resulting in the familiar BVDV syndromes (Nelson et al., 2015; Vilcek and Nettleton, 2006). However, the risk of transmission from wildlife to cattle still needs to be determined (Uzal et al., 2016).
BVDV infection can cause subclinical infections as well as a wide range of particular clinical signs, including diarrhoea, respiratory restlessness, and decreased reproductive performance, including infertility, longer calving intervals, early embryonic death, premature birth, abortive or stillbirth, congenital malformations, as well as persistently infected offspring (PI) (Garoussi et al., 2019 and Timurkan and Aydin 2019).
The virus belongs to the genus Pestivirus, which is within the family Flaviviridae, along with classical swine fever virus and Border disease virus agents, and it has a ssRNA genome of positive polarity with a length of approximately 12.3 kb. A conserved region at the beginning and end of the genome is the untranslated region (UTR). Genes for structural and nonstructural proteins are encoded within a single ORF within the genome (Meyers and Thiel 1996).
International Committee of Taxonomy of Viruses (ICTV) has renamed pestiviruses and classified them into 11 species (Liu et al., 2019). Thus, they are classified as follows: BVDV-1 (Pestivirus A), BVDV-2 (Pestivirus B), border disease (Pestivirus D), classical swine fever (Pestivirus C), and HoBi-like (Pestivirus H). In terms of sub-types, BVDV-1 belongs to the range 1a to 1q, whereas BVDV-2 belongs to the range 2a to 2d., The recent discovery of BVDV-3 (Pestivirus H) of the Pestivirus genus, HoBi-like Pestivirus, has shown that it is genetically and antigenically found in cattle and buffalo (Mishra et al. 2014).
Foetal bovine serum from Brazil was first identified as bearing a HoBi-like Pestivirus in Germany in 2003 (Schirrmeier et al., 2004). The term “HoBi-like Pestivirus” is taken from the first isolate, D32/00 “HoBi.” Several HoBi-like Pestiviruses originated from countries such as South America, Europe, and Asia and were detected in commercial cell culture or FBS batches (Mao et al., 2012; Stahl et al., 2010; Stalder et al., 2005; Xia et al., 2011). The first natural case was reported in 2006 from aborted foetuses in Brazil (Cortez et al., 2006). Since then, several naturally occurring cases of HoBi-like Pestiviruses have been reported in Thailand, Bangladesh, Italy, and Brazil (Decaro et al., 2016; Haider et al., 2014; Liu et al., 2009; Weber et al., 2016; Barreto et al., 2022).
Pestiviruses have been classified according to phylogenetic analysis based primarily on 5′ untranslated regions (5′ UTR) of their genes but also based on genes like Npro, E2, and NS3 (Nagai et al., 2008,and Silveira et al., 2017). According to their cytopathic effect on cell cultures, BVDV species have two biotypes, non-cytopathic (NCP) and cytopathic (CP) (La polla et al., 2022). When NCP viruses are transmitted between the 40th and the 120th day of gestation (vertical transmission), immune-tolerant and PI calves are born (Nelson et al., 2015). The foetal immune system is still immature during this period and unable to distinguish between viral proteins and self-proteins. Some PI calves may live until maturity. If they are retained for breeding, their offspring are always PI but often die during their first year due to secondary infections (Martin et al., 2016).
Since PI animals shed the virus at high titers for life in all their secretions and excretions, they are the primary source of BVDV horizontal transmission in cattle herds (Timurkan and Aydin 2019).
The economic implications of this disease have led several endemic countries, including Egypt, to initiate BVDV control or eradication programs. Many control programs in many countries are based on two main targets: detecting PI animals, removing them from herds, and preventing new PI animals from being introduced with biosecurity programs and/or vaccinations.
BVD viruses are globally important cattle pathogens, with a considerable economic impact due to acute infection and induced immunosuppression, also due to effort needed for control (vaccination, testing, culling). Eradication of BVDV in cattle is widely performed in all cattle-producing countries, including Egypt, but is mainly hindered by the continuous production of PI calves, which consists of a natural reservoir for the virus (Russell et al., 2020).
In this paper, we aim firstly to investigate the prevalence of PI among some dairy cattle herds to identify the main reservoir of the virus as a preliminary step for further institutional molecular-based epidemiological studies of BVDV in Egypt. Therefore, these molecular techniques not only determine the epidemiological status of the infection but also can track the source of infection and contribute to the control of BVDV infection from international trade (Vilček et al., 2003).
Identifying the circulating BVDV genotypes is critical for creating appropriate diagnostic tests and control strategies or vaccinations (Alpay et al., 2019). Therefore, we also aim to identify the current circulating BVDV strain among the cattle population in Egypt.
Materials and methods
Collection and preparation of samples
We collected 240 serum samples from non-vaccinated Holstein calves of different ages (1–6 months) from private dairy cattle farms in six (6) Egyptian provinces (Menya, Beni-suef, Damietta, Fayioum, Kafr-Al sheikh, and Behira) during 2019 and 2020.
The epidemiological data and clinical signs were collected and observed during recurrent farm visits. No specific clinical signs were observed; the case history of the affected farms was retarded growth of many calves, early embryonic death, and subfertility of dams.
To separate serum, a sample set was placed in clean, dry centrifuge tubes, left to clot, and centrifuged at 1500 × g for 20 min. The serum was stored at − 20 °C until further testing.
All tested animals were retested with second samples 5 weeks after the first.
Reference BVD strains
BVDV genotype 1 Egyptian cytopathic strain (Iman strain) and BVDV genotype 2 Egyptian cytopathic strain (Strain 125) were obtained from the Department of Rinderpest like Diseases, Veterinary Serum and Vaccine Research Institute, Cairo, and used as a control strain for RT-PCR testing.
Serological tests
Double Antibody sandwich Elisa assay (Enzyme linked immunosorbent assay) (INGEZIM BVD DAS®) was used for BVDV p80/125 antigen detection in the collected sera samples according to the manufacturing instruction.
BVD p80-125 (NSP2-3) antibodies were detected in sera samples using Competitive Elisa (ID Screen BVD p80 Antibody competition Elisa IDvet Innovative Diagnostic®), as per manufacturer instructions.
For more confirmation about the PI and to avoid errors about TI, these animals were retested 5 weeks apart (Garoussi et al., 2019).
Molecular detection of BVDV
Extraction and RT-PCR
The manufacturer’s instructions were followed when RNA was extracted from 250 μl of each sample and NADL reference strain as a positive control using QIAzol Lysis Reagent (QIAGEN). In brief, each sample was mixed with 750 μl QIAzol reagent and extracted with 150 μl of chloroform. Precipitated viral RNA was prepared using 500 ml isopropanol in the aqueous phase. The next step was to wash the pellet with 750 ml of 75% ethanol and suspend it in 20 ml of sterile DEPC water. As soon as the RNA was extracted, it was reverse transcribed to cDNA using the Revert Aid First Strand cDNA Synthesis kit (Thermo Scientific, Germany) following the manufacturer’s instructions and stored at − 70 °C until it was needed.
A 208 bp fragment of the 5′ untranslated region (5′ UTR) of the Pestivirus genome was amplified by PCR amplification by BVD 190-F and V326 primer pairs (Moorthy et al., 2019) for the detection of BVDV in a 25 *L reaction volume. A total of 12.5 μl (2X) Thermo Scientific™ DreamTaq™ Green PCR Master Mix (Thermo Scientific, Germany) was used in each reaction; 0.25 μl (25 pmol) BVD 190-F forward primer (5′-GRAGTCGTCARTGGTTCGAC-3′), 0.25 μl (25 pmol) V326 reverse primer (5′-TCAACTCCATGTGCCATGTAC-3′), 7 μl water, and 5 μl cDNA sample. The PCR tubes were placed in the Thermo Scientific™ PCR detection system, which programmed for the test as follows: 4 min at 95 °C, then 35 cycles of denaturation step at 94 °C for 45 s, annealing at 58 °C for 45 s and extension at 72 °C for 45 s followed by one cycle of final extension at 72 °C for 10 min. Analysis of PCR products was carried out in 2.0% agarose gel electrophoresis and visualized on a UV trans-illuminator.
Sequencing and phylogenetic analysis
QIAquick Gel Extraction Kit (Qiagen) was used to purify positive amplicons (in terms of the 5′ UTR gene) from the gel as instructed by the manufacturer. ABI PRISM Big Dye Terminators v3.1Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) was used to directly sequence the purified PCR products. Applied Bio-Systems, CA-USA, provided a Centrisep purification kit to clean up the sequencing reaction products. ABI PRISM3500genetic analyzer (Applied Biosystems) was used to sequence the purified products directly.
To identify the sequence identity, BLAST® analysis (Basic Local Alignment Search Tool) was performed (Asplund et al., 2019). In BioEdit Program v.7.0.5 (Hall 1999), raw sequence reaction data were aligned with reference strains obtained from Genbank. In order to test the significance of the phylogenetic tree, we constructed it with the maximum likelihood (ML) method and performed a bootstrap test (1000 replication) using Mega version 6 (Tamura et al., 2013).
Results
Detection of PI state in collected samples
All samples were serologically tested to detect BVD, both antigen and antibodies, to investigate the PI state among tested animals. Samples negative for antibodies and positive for antigen (tested twice 5 weeks apart) indicated PI animal. Six (2.5%) of two hundred and forty studied samples (6/240) were found to be PI with BVDV (Table 1).
Molecular detection of BVDV
Six samples of BVDV PI animals were tested for molecular confirmation using oligonucleotide primers specific to the BVDV 5′ UTR described previously. The gene of the 5′ UTR was amplified, and all samples were found positive to have a specific M.W band at 208 bp.
Sequence and sequence analysis
Partial nucleotide sequence of the 5′ UTR gene of six tested samples named: (BVD-3_AHRI_EGY_1, BVD-3_AHRI_EGY_2, BVD-3_AHRI_EGY_3, BVD-3_AHRI_EGY_4, BVD-3_AHRI_EGY_5, and BVD-3_AHRI_EGY_6) has been submitted to the Genbank with accession numbers (OM324396, OM324397, OM324398, OM324399, OM3243100, and OM3243101) respectively.
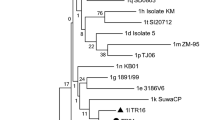
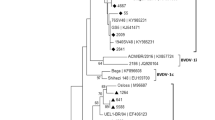
Based on the phylogenetic analysis, all six samples were atypical HoBi-like Pestiviruses (BVD-3). According to the analysis of sequence data, our strains are 99.6% similar to the Brazilian BVD-3 reference strain (ACC. KY683847), 98.8% identity with the BVD-3 reference strain from Italy (ACC. MH410816), and 99.6% identity with BVD-3 other reference strain from Italy (ACC. HQ231763) (Fig. 1 and Table 2).
Discussion
During this study, we aimed to investigate the prevalence of PI state among some Egyptian dairy cattle herds and to detect and identify the current strain circulating among the national cattle population.
The PI calves were detected by serological analysis of 240 serum samples. These samples were tested to identify seronegative (immunotolerant) animals which are positive at the same time for the BVDV antigen for two successive tests. This came in agreement with previous records, which revealed that persistently infected animals do not produce BVDV-specific antibodies (Dezen et al., 2013; Alpay et al., 2019).
In the present study, the size of the unvaccinated herd is medium-sized, and examining each animal is not strategically or financially solution for PI animals’ detection.
When seronegative dairy animals are naturally infected, no extreme illness is caused by BVDV; they have become transiently infected (TI) animals with antigens that may be detected for weeks (Niskanen and Lindberg 2003). TI animals may be a source of horizontal infection, as it is previously reported that BVDV can survive in a herd even when PI animals are absent (Moennig et al., 2005). PI and TI animals likely existed in this study, and due to the greater probability of BVD virus shedding in PI than the TI cows, the PI cows were considered the leading cause of infection in this study. For more confirmation about the PI and to avoid errors about TI, these animals were retested 5 weeks apart (Garoussi et al., 2019).
The PI calves with BVDV in the present study were detected serologically and confirmed with RT-PCR in six out of two hundred and forty studied samples (6/240), the same results reported previously by Hilbe and co-authors. They detected three PI animal cases in their research using ELISA and RT-PCR techniques for testing serum samples collected from calves infected by BVDV (Hilbe et al., 2007).
The worldwide pooled PI prevalence at the animal level ranged from low (≤ 0.8% Europe, North America, and Australia), medium (> 0.8 to 1.6% East Asia) to high (> 1.6% West Asia) (Scharnböck et al., 2018).
According to the current study’s findings, the prevalence of PI animals was 2.5%. A high percentage of seropositive animals (32.08%) indicated the presence of PI animals within the herd (Garoussi et al., 2019). Some studies reported that the BVDV PI prevalence in cattle was lower and extended from 0.5 to 2% (Brock, 2003; Peterhans et al., 2003; Smith et al., 2008). Thus, it is clear that the prevalence of PI within herds is variable, and it can reach as high as 30–35% when many naïve cows are exposed to NCP BVDV early in pregnancy (Khodakaram-Tafti et al., 2017). On the other hand, the prevalence of calves being born PI thus diminished substantially from around 1.4 to < 0.02%.in Switzerland (Schweizer et al., 2021).
Moreover, comparing the situation in Egypt, collectively, we found that the current study PI prevalence is lower compared to the latest study, where the PI was confirmed in 9 calves out of 305 calves (2.95%) in Damietta governorate in Egypt (Atwa et al., 2019). An earlier study also detected that BVDV prevalence is 8.4% of serum samples (21/250) by antigen capture ELISA (El-Bagoury et al., 2014).
Such discrepancy in the reported prevalence percentages may be due to many factors: for instance, the used diagnostic tests in each study, the variation in the management system for the examined herd, and the locations of the examined population (Atwa et al., 2019).
Moving to another factor, the herding breed might play a significant role in determining BVDV prevalence. It should be noted that most of the cattle examined in this study were pure Holsteins. As previously reported, the prevalence of BVDV in pure Holstein Friesian cows was highest (46.6%) compared to Brown Swiss (21.8%) and Creole breeds (22.7%) (Herrera-Yunga et al., 2018). Hence, the genetic factor affected cow susceptibility to BVDV infection (Ortega et al., 2020).
Considering the age factor, the PI prevalence in this study was observed in calves ranging from 1 to 6 months, which came following Smirnova et al. (2008), who revealed that at birth, the prevalence of BVDV PI is highest, and it declines with age.
As a routine diagnostic method for BVDV, RT-PCR has gained widespread use in the past decade (Smith et al., 2008). It has been shown that PCR allows for the detection of BVDV in a variety of clinical samples such as serum, buffy coats, tissues, foetal fluids, milk, and nasal swabs (Renshaw et al., 2000;Stokstad et al., 2003;Kennedy et al., 2006;Youngl et al., 2006;Edmondson et al., 2007;Tajima et al., 2008;Khodakaram-Tafti et al., 2016). This technique is reliable for diagnosing BVDV in all ages of PI, even with maternal antibodies to BVDV that affect the accuracy of other tests such as VI and ELISA (Luzzago et al., 2001; Saliki and Dubovi, 2004; Goyal, 2005; Sandvik, 2005).
Regarding genome organization of the virus, the BVDV E2 structural protein induces the production of neutralizing antibodies (Thomas et al., 2009), and the Npro nonstructural protein contributes to virulence (Darweesh et al., 2018). Pestivirus strains are frequently classified using the 5′UTR. Interestingly, the new strain BVD-3 AHRI EGY detected in this study shared more homology in the 5′UTR with other strains from Brazil and Italy. This new emerging strain may be introduced to Egypt from those areas through cattle importations and also may be due to the trading of contaminated biological products (Bauermann et al., 2013).
Even though there was not enough information about the presence of HoBi-like Pestivirus in the Middle East and African countries, there were some previous reports about the widespread presence of BVDV-1 and sporadic existence of BVDV-2 (El-bahgy et al., 2018;Atwa et al., 2019;Lotfy et al., 2020;Guidoum et al., 2020;Pawlos et al., 2020; Aboezz et al., 2021 and Endeshaw et al., 2021).
HoBi-like Pestivirus infections have recently posed a concern for cattle and small ruminants in South America, Europe, and Asia. To our knowledge, there have been no clinical cases of HoBi-like Pestivirus infection in cattle in the Middle East, although the discovery of HoBi-like Pestivirus strains in contaminated cell cultures, commercial FBS, and small ruminants.
In conclusion, the current study successfully identified a new emerging atypical HoBi-like Pestivirus (BVD-3) among PI cattle herds for the first time in Egypt.
This study has some limitations. The BVDV PIs have been identified in bovine calves reared in only six Egyptian provinces and did not elucidate PI in other provinces. Consequently, it is necessary to carry out further studies to investigate the PI of BVDV in other provinces, particularly the southern part of Egypt and the delta. Additional studies are required to identify BVDV infection in other animal species susceptible to BVDV, such as buffalo calves and goats. This will help to establish a strategy for controlling BVD infection.
It was revealed that there is a correlation found between the prevalence of seropositive animals and the presence of BVD PI within the herds. Overall, to minimize the diagnostic detection costs and economic losses caused by this disease, it is recommended to pool whole blood samples for RT-PCR.
To evaluate the outcome of animal genetics on both individual and herd seroprevalences levels, more future studies with enough details focusing on the type of production system utilized by the sampled animals and their breed composition are required.
Further molecular-based analysis on 5′ UTR complete gene sequence or BVDV entire genome sequence by NGS (next generation sequencing) would investigate the evolution of quasispecies of the identified BVD strain as a preliminary step for control of PI in Egypt.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Aboezz, Z., elnagar, S., El-Mohamady, R., El-Habbaa, A., and El Nahas, E. (2021): Isolation and Identification of Bovine Viral Diarrhea Virus from Ovaries of Egyptian heifer and cow in 2019. Benha Veterinary Medical Journal, 41(1), 42-44.
Alpay, G., Toker, E.B. and Yeşilbağ, K. (2019): Persistent BVD virus infections in offspring from imported heifers. Trop Anim Health Prod 51, 297–302.
Asplund, M., Kjartansdóttir, K. R., Mollerup, S., Vinner, L., Fridholm, H., Herrera, J. A., and Hansen, A. J. (2019): Contaminating viral sequences in high-throughput sequencing viromics: a linkage study of 700 sequencing libraries. Clinical Microbiology and Infection, 25(10), 1277-1285.
Atwa, S.M., Emad, Y.E., Rizk, M.A. (2019): An epidemiological survey of bovine viral diarrhoea infection in calves in Egypt with identification of high prevalence of persistently infected animals. Comp Clin. Pathol. 28, 447–453.
Barreto JVP, Lorenzetti E, Fritzen JTT, Jardim AM, Oliveira TES, Headley SA, Alfieri AA, da Cunha Filho LFC. (2022): Congenital Neurological Disease Associated with HoBi-like Pestivirus Infection in a Newborn Dairy Calf From Brazil. Front Vet Sci. 24; 9:852965.
Bauermann, F. V., Ridpath, J. F., Weiblen, R., and Flores, E. F. (2013): HoBi-like viruses: An Emerging group of Pestiviruses. Journal of Veterinary Diagnostic Investigation, 25(1), 6–15.
Brock, KV (2003): Bovine viral diarrhoea virus persistence. Biologicals. 31: 133-135.
Cortez, A., Heinemann, M. B., Castro, A. M. M. G. D., Soares, R. M., Pinto, A. M. V., Alfieri, A. A., Flores, E. F., Leite, R. C., and Richtzenhain, L. J. (2006): Genetic characterization of Brazilian bovine viral diarrhea Virus isolates by partial nucleotide sequencing of the 5'-UTR region. Pesquisa Veterinária Brasileira, 26(4), 211–216.
Darweesh, M. F., Rajput, M. K. S., Braun, L. J., Rohila, J. S., and Chase, C. C. L. (2018): BVDV Npro protein mediates the BVDV induced immunosuppression through interaction with cellular S100A9 protein. Microbial Pathogenesis, 121, 341–349.
Daves, L., Yimer, N., Arshad, S.S., Sarsaifi, K., Ariff, M., Yusoff, R., Wahid, A., and Jesse F.F. (2016): Seroprevalence of bovine viral diarrhoea virus (BVDV) infection and associated risk factors in cattle in Selangor, Malaysia. Open J. Vet. Med., 1(1): 22-28.
Decaro, N., Lucente, M. S., Losurdo, M., Larocca, V., Elia, G., Occhiogrosso, L., and Buonavoglia, C. (2016): HoBi-like Pestivirus and its impact on cattle productivity. Transboundary and Emerging Diseases, 63(5), 469– 473.
Dezen S, Otonel RAA, Aleri AF, Lunardi M, and Aleri AA (2013): Bovine viral diarrhoea virus (BVDV) infection prole in a high production dairy herd with vaccination program against BVDV. Pesqui Vet Bras 33:141147.
Edmondson, MA; Givens, MD; Walz, PH; Gard, JA; Stringfellow, DA and Carson, RL (2007): Comparison of tests for detection of bovine viral diarrhoea virus in diagnostic samples. J. Vet. Diagn. Invest, 19: 376-381.
El Bahgy, H., Abdelmegeed, H. K., and Marawan, M. A. (2018): Epidemiological surveillance of bovine viral diarrhoea and rift valley fever infections in camel. Veterinary World, 11(9), 1331–1337.
El-Bagoury GF, El-Nahas EM, El-Deen SSS, and Salem SAH (2014): Detection and genotyping of bovine viral diarrhoea virus in cattle sera. Benha Vet Med J 27:348–353.
Endeshaw Demil, Tsegaw Fentie, Gema Vidal, Wendi Jackson, Jennifer Lane, Sefinew Alemu Mekonnen, and Woutrina Smith. (2021): Prevalence of bovine viral diarrhoea virus antibodies and risk factors in dairy cattle in Gondar city, Northwest Ethiopia, Preventive Veterinary Medicine, Volume 191, 105363, ISSN 0167-5877,
Garoussi, M. T., Mehrzad, J., and Nejati, A. (2019): Investigation of persistent infection of bovine viral diarrhoea virus (BVDV) in Holstein dairy cows. Tropical animal health and production, 51(4), 853-858.
Goyal, SM (2005): Diagnosis. In: Goyal, SM, and Ridpath, JF (Eds.), Bovine viral diarrhoea virus: diagnosis, management, and control. (1st Edn.), Ames, IA, Blackwell Publishing. PP: 197–208.
Guidoum, K.A., Benallou, B. , Pailler, L. , Espunyes, J., Napp, S. and Cabezón, O. (2020): Ruminant Pestiviruses in North Africa, Preventive Veterinary Medicine, Volume 184, 105156, ISSN 0167-5877.
Haider, N., Rahman, M.S., Khan, S.U., Mikolon, A., Gurley, E.S., Osmani, M.G., Shanta, I.S., Paul, S.K., Macfarlane-Berry, L., Islam, A., Desmond, J., Epstein, J.H., Daszak, P., Azim, T., Luby, S.P., Zeidner, N. and Rahman M.Z., (2014): Identification and epidemiology of a rare HoBi-like Pestivirus strain in Bangladesh. Transboundary and Emerging Diseases, 61 (3), 193–198.
Hall, T.A., (1999): BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Herrera-Yunga, V., Labada, J., Castillo, F., Torres, A., Escudero-Sanchez, G., Capa-Morocho, M., & Abad-Guaman, R. (2018). Prevalence of antibodies and risk factors to bovine viral diarrhoea in non-vaccinated dairy cattle from Southern Ecuador. Tropical and Subtropical Agroecosystems, 21(1).
Hilbe M, Stalder H, Peterhans E, Haessig M, Nussbaumer M, Egli C, Schelp C, Zlinszky K, and Ehrensperger F (2007): Comparison of five diagnostic methods for detecting bovine viral diarrhoea virus infection in calves. J Vet Diagn. Investig. 19:28–34.
Kennedy, JA; Mortimer, RG, and Powers, B (2006): Reverse transcription-polymerase chain reaction on pooled samples to detect bovine viral diarrhoea virus using fresh ear notch-sample supernatants. J. Vet. Diagn. Invest, 18: 8993.
Khodakaram-Tafti, A; Mohammadi, A and Farjanikish, GH (2016): Molecular characterization and phylogenetic analysis of bovine viral diarrhoea virus in dairy herds of Fars province, Iran. Iran. J. Vet. Res., 17: 89-97.
Khodakaram-Tafti A, and Farjanikish GH (2017): Persistent bovine viral diarrhoea virus (BVDV) infection in cattle herds. Iran J Vet Res 18:154-163.
La Polla, R., Testard, M. C., Goumaidi, A., Chapot, E., Legras-Lachuer, C., & de Saint-Vis, B. (2022). Identification of differentially expressed gene pathways between cytopathogenic and non-cytopathogenic BVDV-1 strains by analysis of the transcriptome of infected primary bovine cells. Virology, 567, 34–46.
Liu, C., Liang, L., & Cui, S. (2019). Modification for Pestivirus in the tenth report of ICTV. Chinese Journal of Virology, 35(3), 565–568.
Liu, L., Kampa, J., Belak, S., and Baule, C. (2009): Virus recovery and full-length sequence analysis of atypical bovine Pestivirus Th/04_KhonKaen. Veterinary Microbiology, 138(1–2), 62–68.
Lotfy, A., Selim, A., Ibrahim, A., and Salem, S. (2020): Seroprevalence and Molecular characterization of Bovine viral diarrhoea, Rota and coronaviruses in neonatal cattle and buffalo calves in some governorates in Egypt. Benha Veterinary Medical Journal, 38(2), 5-9.
Luzzago, C; Bandi, C; Bronzo, V; Ruffo, G, and Zecconi, A (2001): Distribution pattern of bovine viral diarrhoea virus strains in intensive cattle herds in Italy. Vet. Microbiol. 26: 265-274.
Mao, L., Li, W., Zhang, W., Yang, L., and Jiang, J. (2012): Genome sequence of a novel Hobi-like Pestivirus in China. Journal of Virology, 86(22), 12444.
Martin CC, Baccili CC, Silva BT, Novo SMF, Sobreira NM, Pituco EM, and Gomes V (2016): Detection of Bovine Viral Diarrhea virus infection in newborn calves before colostrum intake. Semina 37:1379-1388.
Meyers, G., and Thiel, H.J., (1996): Molecular characterization of Pestiviruses, Advances in Virus Research, 47, 53–118.
Mishra, N., Rajukumar, K., Pateriya, A., Kumar, M., Dubey, P., Behera, S.P., Verma, A., Bhardwaj, P., Kulkarni, D.D., Vijaykrishna, D., and Reddy, N.D., (2014): Identification and molecular characterization of novel and divergent HoBi-like Pestiviruses from naturally infected cattle in India, Veterinary Microbiology, 174 (1–2), 239–246.
Moennig, V., Houe, H., and Lindberg, A., (2005): BVD control in Europe: current status and perspectives. Animal Health Research Reviews. 6, 63–74.
Moorthy, D., Mishra, N., Kalaiyarasu, S., Jhade, S. K., & Singh, V. P. (2019): Evaluation of currently available bovine viral diarrhoea virus (BVDV) and HoBi-like Pestivirus (HoBiPeV) specific diagnostic tests in the detection of highly divergent HoBiPeVs in cattle. Journal of virological methods, 272, 113707.
Nagai, M., Hayashi, M., Itou, M., Fukutomi, T., Akashi, H., Kida, H., and Sakoda, Y. (2008). Identification of new genetic subtypes of bovine viral diarrhoea virus genotype 1 isolated in Japan. Virus Genes, 36(1), 135-139.
Nelson, DD; Duprau, JL; Wolff, PL, and Evermann, JF (2015): Persistent bovine viral diarrhoea virus infection in domestic and wild small ruminants and camelids including the mountain goat (Oreamnos americanus). Front Microbial., 6: 1415-1422.
Niskanen, R., and Lindberg, A., (2003): Transmission of bovine viral diarrhoea virus by unhygienic vaccination procedures, ambient air, and contaminated pens. Veterinary Journal. 165(2):125–30.
Ortega DO, Martinez R, Tobón JC, and Rocha JF (2020): Prevalence and risk factors of bovine viral diarrhoea in Colombian cattle, Veterinary World, 13(8): 1487-1494.
Pawlos Asnake, Abdissa Lemma, and Asamnew Tesfaye. (2020): Sero-prevalence of Bovine Viral Diarrhea Virus (BVDV) and Its Associated Risk Factors in Dairy Cattle in and Around Assela Town, South East Ethiopia.
Peterhans, E; Jungi, TW and Schweizer, M (2003): BVDV and innate immunity. Biologicals. 31: 107-112.
Renshaw, RW; Ray, R, and Dubovi, EJ (2000): Comparison of virus isolation and reverse transcription-polymerase chain reaction assay for detection of bovine viral diarrhoea virus in bulk milk tank samples. J. Vet. Diagn. Invest., 12: 184-186.
Richter V, Kattwinkel E, Firth C, Marschik T, Dangelmaier M, Trauffler M, Obritzhauser W, Baumgartner W, Käsbohrer A, and Pinior B. (2019): Mapping the global prevalence of bovine viral diarrhea virus infection and its associated mitigation programmes. Vet. Rec.; 184 (23):711.
Russell, G. C., Zadoks, R. N., Willoughby, K., and Bachofen, C. (2020): Bovine viral diarrhoea virus loses quasispecies diversity rapidly in culture. Microbial genomics, 6(4).
Saliki, JT and Dubovi, EJ (2004): Laboratory diagnosis of bovine viral diarrhoea virus infections. Vet. Clin. North Am.: Food Anim. Pract., 20: 69-83.
Sandvik, T (2005): Selection and use of laboratory diagnostic assays in BVD control programs. Preven. Vet. Med., 72: 3-16.
Scharnböck, B., Roch, F. F., Richter, V., Funke, C., Firth, C. L., Obritzhauser, W., and Pinior, B. (2018): A meta-analysis of bovine viral diarrhoea virus (BVDV) prevalences in the global cattle population. Scientific reports, 8(1), 1-15.
Schirrmeier, H., Strebelow, G., Depner, K., Hoffmann, B., and Beer, M., (2004): Genetic and antigenic characterization of an atypical Pestivirus isolate, a putative member of a novel Pestivirus species, Journal of General Virology, 85, 3647–3652.
Schweizer, M., Stalder, H., Haslebacher, A., Grisiger, M., Schwermer, H., & Di Labio, E. (2021): Eradication of bovine viral diarrhoea (BVD) in cattle in Switzerland: Lessons taught by the complex biology of the virus. Frontiers in veterinary science, 1012.
Silveira, S., Weber, M. N., Mósena, A. C. S., Da Silva, M. S., Streck, A. F., Pescador, C. A., and Canal, C. W. (2017). Genetic diversity of Brazilian bovine Pestiviruses was detected between 1995 and 2014. Transboundary and Emerging Diseases, 64(2), 613-623.
Smirnova, NP; Bielefeldt-Ohmann, H; Van Campen, H; Austin, KJ; Han, H; Montgomery, DL; Shoemaker, ML; van Olphen, AL and Hansen, TR (2008): Acute noncytopathic bovine viral diarrhoea virus infection induces pronounced type I interferon response in pregnant cows and fetuses. Virus Res., 132: 49-58.
Smith, RL; Sanderson, MW; Walz, PH and Givens, MD (2008): Sensitivity of polymerase chain reaction for detection of bovine viral diarrhoea virus in pooled serum samples and use of pooled polymerase chain reaction to determine the prevalence of bovine viral diarrhoea virus in auction market cattle. J. Vet. Diagn. Invest, 20: 75-78.
Stahl, K., Beer, M., Schirrmeier, H., Hoffmann, B., Belak, S., and Alenius, S. (2010): Atypical 'HoBi'-like Pestiviruses–recent findings and implications thereof Veterinary Microbiology, 142(1–2), 90–93.
Stalder, H. P., Meier, P., Pfaffen, G., Wageck-Canal, C., Rufenacht, J., Schaller, P., and Peterhans, E. (2005): Genetic heterogeneity of Pestiviruses of ruminants in Switzerland. Preventive Veterinary Medicine, 72(1-2), 37–41; Discussion 215–219.
Stokstad, M; Niskanen, R; Lindberg, A; Thoren, P; Belak, S; Alenius, S and Loken, T (2003): Experimental infection of cows with bovine viral diarrhoea virus in early pregnancy – findings in serum and fetal fluids. J. Vet. Med., 50: 424-429.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S., (2013): MEGA6: molecular evolutionary genetics analysis version 6.0, Molecular Biology and Evolution, 30, 2725–2729.
Tajima, M; Ohsaki, T; Okazawa, M and Yasutomi, I (2008): Availability of oral swab sample for the detection of bovine viral diarrhoea virus (BVDV) gene from the cattle persistently infected with BVDV. Jpn. J. Vet. Res., 56: 3-8.
Thomas, C., Young, N. J., Heaney, J., Collins, M. E., and Brownlie, J. (2009): Evaluation of efficacy of mammalian and Baculovirus expressed E2 subunit vaccine candidates to bovine viral diarrhoea virus. Vaccine, 27(17), 2387–2393.
Timurkan MO, and Aydin H (2019): Increased genetic diversity of BVDV strains circulating in Eastern Anatolia, Turkey: first detection of BVDV-3 in Turkey. Trop Anim Health Prod 51:1953-1961.
Uzal, FA; Plattner, BL, and Hostetter, JM (2016): Alimentary system in pathology of domestic animals. In: Maxie, MG (Ed.), Jubb, Keneddy and Palmers pathology of domestic animals. (6th Edn.), Vol. 2, St. Louis, Missouri, Academic Press Inc., P.P: 122–130.
Vilček, Š., Mojžišová, J., Bajová, V., Paulík, Š., Strojný, L., Ďurkovič, B., and Hipíková, V. (2003): A survey for BVDV antibodies in cattle farms in Slovakia and genetic typing of BVDV isolates from imported animals. Acta Veterinaria Hungarica, 51(2), 229-236.
Vilcek, S, and Nettleton, (2006): Pestiviruses in wild animals. Vet. Microbiol. 116: 1-12.
Weber, M. N., Mosena, A. C., Simoes, S. V., Almeida, L.L., Pessoa, C. R., Budaszewski, R. F., and Canal, C. W. (2016): Clinical presentation resembling mucosal disease associated with 'HoBi'-like Pestivirus in a field outbreak. Transboundary and Emerging Diseases, 63(1), 92–100.
Xia, H., Vijayaraghavan, B., Belak, S., and Liu, L. (2011): Detection and identification of the atypical bovine Pestiviruses in commercial fetal bovine serum batches. PLoS One, 6(12), e28553.
Youngl, NJ; Thomas, CJ; Collins, ME and Brownlie, J (2006): Real-time RT-PCR detection of bovine viral diarrhoea virus in whole blood using an external RNA reference. J. Virol. Methods. 138: 218-222.
Acknowledgements
The authors would like to extend their appreciation and thanks to Animal Health Research Institute, Agriculture Research Center, Egypt, for assistance and for providing all laboratory facilities till the completion of this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to analyzing data, final revision, and approving submission. AFA and RTH: contributed to the study conception and design. HKA, MHA, and DAA: carried out serological testing, material preparation, and data collection. TSB and EAA: carried out molecular identification. AFA: wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
As biological samples were received from an authorized government agency (Animal Health Research Institute, Agricultural Research Centre, Egypt), this study did not require ethical approval. Animal ethics procedures and guidelines for the Arab Republic of Egypt demanded that samples be collected and handled in line with good animal practices.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afify, A.F., Hassanien, R.T., Abdelmegeed, H.K. et al. First detection of emerging HoBi-like Pestivirus (BVD-3) among some persistently infected dairy cattle herds in Egypt. Trop Anim Health Prod 54, 336 (2022). https://doi.org/10.1007/s11250-022-03332-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03332-2