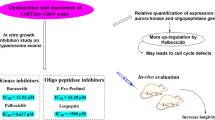
Abstract
High cytotoxicity and increasing resistance reports of existing chemotherapeutic agents against T. evansi have raised the demand for novel, potent, and high therapeutic index molecules for the treatment of surra in animals. In this regard, repurposing approach of drug discovery has provided an opportunity to explore the therapeutic potential of existing drugs against new organism. With this objective, the macrocyclic lactone representative, ivermectin, has been investigated for the efficacy against T. evansi in the axenic culture medium. To elucidate the potential target of ivermectin in T. evansi, mRNA expression profile of 13 important drug target genes has been studied at 12, 24, and 48 h interval. In the in vitro growth inhibition assay, ivermectin inhibited T. evansi growth and multiplication significantly (p < 0.001) with IC50 values of 13.82 μM, indicating potent trypanocidal activity. Cytotoxicity assays on equine peripheral blood mononuclear cells (PBMCs) and Vero cell line showed that ivermectin affected the viability of cells with a half-maximal cytotoxic concentration (CC50) at 17.48 and 22.05 μM, respectively. Data generated showed there was significant down-regulation of hexokinase (p < 0.001), ESAG8 (p < 0.001), aurora kinase (p < 0.001), casein kinase 1 (p < 0.001), topoisomerase II (p < 0.001), calcium ATPase 1 (p < 0.001), ribonucleotide reductase I (p < 0.05), and ornithine decarboxylase (p < 0.01). The mRNA expression of oligopeptidase B remains refractory to the exposure of the ivermectin. The arginine kinase 1 and ribonucleotide reductase II showed up-regulation on treatment with ivermectin. The ivermectin was found to affect glycolytic pathways, ATP-dependent calcium ATPase, cellular kinases, and other pathway involved in proliferation and maintenance of internal homeostasis of T. evansi. These data imply that intervention with alternate strategies like nano-formulation, nano-carriers, and nano-delivery or identification of ivermectin homologs with low cytotoxicity and high bioavailability can be explored in the future as an alternate treatment for surra in animals.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The extracellular hemoflagellate, Trypanosoma evansi, has a wide spectrum of hosts and is responsible for surra in animals (Desquesnes et al. 2013). Surra is a debilitating and economically significant illness of equines, bovines, and camelids, which is extensively dispersed in parts of Africa, Asia, and South America (Radwanska et al. 2018; Aregawi et al. 2019; Yadav et al. 2019; Sharma et al. 2022). Chemotherapy forms the mainstay for treatment and control of surra in animals (Giordani et al. 2016). However, limited number of drugs, high toxicity, narrow therapeutic index, and emerging resistance has raised the requirement of alternate therapeutic for treatment and control of T. evansi infection in animals. Drug repurposing is a new strategy to therapeutic discovery that cuts down the time and money spent on identifying and developing new drug molecules (Pushpakom et al. 2019; Rudrapal et al. 2020).
The macrocyclic lactone, ivermectin, was the first endectocide drug, eliminating a wide range of ecto-parasites (Brooks and Grace 2002), helminths, and protozoan parasites (Panchal et al. 2014; Mendes et al. 2017; Omura and Crump 2017; Pinilla et al. 2018; Batiha et al. 2019) both inside and outside the body (Canga et al. 2008). Later, the drug has surprised the world with its antitumor (Dou et al. 2016), antibacterial (Omura and Crump 2014), and antiviral (Caly et al. 2020) activity. Recently, the ivermectin has been documented to be effective against B. bovis, B. bigemina, B. divergens, B. caballi, and T. equi with half-maximal inhibitory concentration (IC50) in the micromolar (30.1–98.6) range (Batiha et al. 2019).
Ivermectin has also been investigated against several kinetoplastid parasites. In dogs, experimentally infected with T. cruzi, ivermectin treatment did not show any apparent effect on Trypanosoma infection (Dias et al. 2005). In 2012, 300 μg/mL/kg dose of ivermectin was reported to increase the mean survival period of T. brucei brucei-infected mice from 5 to 12 days (Udensi and Fagbenro-Beyioku 2012). Later, it was reported that ivermectin has no chemotherapeutic effect against T. b. brucei infection in rats (Osondu et al. 2016). Nowadays, several researchers classify T. evansi and T. equiperdum as the subspecies of T. brucei (Cuypers et al. 2017; Oldrieve et al. 2021). Recently, ivermectin was found to be effective against Leishmania infantum under in vitro and in vivo system as a potential therapeutic compound against visceral leishmaniasis (Reis et al. 2021). There was lack of information regarding activity of ivermectin against T. evansi. With this research gap, the ivermectin has been investigated in this study to evaluate its trypanocidal activity against T. evansi. Further, the mRNA expression profile of several important drug target genes of T. evansi on exposure to ivermectin was studied to un-fold the changes in cellular environment caused by the drug.
Materials and methods
Propagation of Trypanosoma evansi in culture medium
The cryostabilate of equine T. evansi isolate (T. ev-India-NRCE-Horse1/Hisar/Haryana), maintained at Parasitology Laboratory, ICAR-National Research Institute on Equines (India), was used in the present study. The cryostabilate was thawed, viability of T. evansi was checked, and numbers of parasites were counted in Neubauer hemocytometer chamber (Improved Neubauer, depth 01 mm, 1/400 mm2, ROHEM, India) and intraperitoneally inoculated (1 × 105 T. evansi) in Swiss albino mice (6–8 weeks old, female) obtained from Disease Free Small Animal House, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, India, as described by Baltz et al. (1985). At peak parasitemia (108 trypanosomes/mL), 4–5 days post-infection, trypanosomes were purified from mice blood as described by Kumar et al. (2015). Prior approval for animal experimentation was taken from Institutional Animal Ethics Committee (IAEC) of ICAR-NRCE, Hisar [Regn No. 193/GO/Re/SL/99/CPCSEA]. Purified parasites were cultivated in HMI-9 medium prepared by supplementing Iscove’s Modified Dulbecco’s Medium (IMDM) with 100 μM bathocuproic acid, 0.001% holotransferrin, 1 mM sodium pyruvate, 100 μM hypoxanthine, 16 μM thymidine, 2 μM mercaptoethanol, 1.94 μM L-cysteine, 60 μM HEPES, 4 mM L-glutamine, 0.4% BSA, 1% antibiotics (1 mL penicillin 10,000 IU/mL-streptomycin 10 mg/mL solution in 100 mL medium), and 20% fetal bovine serum (FBS, GIBCO, USA) (Hirumi and Hirumi 1989). All the chemicals mentioned above were procured from Sigma-Aldrich (USA). Then, the parasites were adapted and maintained in HMI-9 medium at 37 °C and 5% CO2 atmosphere in CO2 incubator (New Brunswick™, Galaxy® 170 R, Eppendorf AG, Germany) for the in vitro drug efficacy, cytotoxicity studies, and transcript analysis.
In vitro growth inhibition assay
Ivermectin (Hitek, Virbac, France) was used as a drug molecule in present study. Stock solutions of ivermectin were prepared in dimethyl sulphoxide (DMSO). Ivermectin at 1 µM, 5 µM, 10 µM, 20 µM, 40 µM, 50 µM, and 100 µM concentration was added at 24, 48, and 72 h in HMI-9 medium. Quinapyramine methyl sulphate (QPS, TriquinS™, Vetoquinol India Animal Health Pvt. Ltd.) at concentration of 20 µM was used as the standard drug. From the well-adapted trypanosome culture, a parasite density of 1 × 105 cells/mL was prepared by adding fresh HMI-9 medium and 500 µL each was seeded on a 48 well plate (Greiner Bio-One, Cellstar). The experiments were carried out in triplicate wells for each concentration of drugs and drugs were added at the 24 h interval in culture medium. Parasite culture without any drug molecule was kept as negative control. Parasite culture with 1% DMSO (Sigma-Aldrich, USA) and 20 µM QPS was taken as solvent control and positive control, respectively. The plate was incubated in CO2 incubator (5% CO2, 95% air) at 37 °C for a period of 72 h. The culture was monitored every 24 h up to 72 h post drug inoculation under phase contrast inverted microscope (Olympus, Japan) and parasite density was counted using optimized CellTiter-Glo® luminescent cell viability assay reagent (Promega, USA). The 50% inhibitory concentration (IC50) was calculated at 24 h by interpolation using the curve fitting technique in GraphPad Prism software (version 8.0.2). The non-linear regression analysis curve was prepared based on result of luciferase assay by plotting percentage (%) growth inhibition of T. evansi against the varied concentrations of the drug as per the standard guidelines (Seabaugh 2011).
Vero cell line and equine PBMCs cytotoxicity assays
Cytotoxicity assays on Vero cell line and equine PBMCs were performed as previously described with slight modifications (Kumar et al. 2020). Vero cell line was maintained in Eagle’s minimum essential medium (EMEM, Sigma-Aldrich, USA) in CO2 incubator (New Brunswick™, Galaxy® 170 R, Eppendorf AG, Germany) at 37 °C. For determination of cytotoxic activity of ivermectin, 1 × 104 Vero cells were seeded in 96 well plates and incubated at 37 °C in the presence of 5% CO2 for 24 h. Similarly, equine PBMCs were isolated from equine venous blood by differential centrifugation over Ficoll Paque™ Premium 1.073 (GE Healthcare Bio-Science AB, Sweden). The cells were re-suspended in RPMI-1640 (Sigma-Aldrich, USA) complete medium (10% fetal bovine serum, 1 mL penicillin 10,000 IU/mL-streptomycin 10 mg/mL solution), enumerated using a Neubauer hemocytometer, and 2 × 105 cells were seeded in triplicate in a 96 well plate (Greiner Bio-One, Cellstar). The PBMCs culture was activated using Phytohemagglutinin-L (PHA-L, Roche Diagnostic, Germany) at 10 µg/mL for 24 h and incubated in CO2 incubator at 37 °C with 5% CO2. After incubation, the Vero cells and equine PBMCs were treated with different concentrations of ivermectin (13, 26, 65, 130, and 260 µM) in triplicate well and incubated in CO2 incubator for 48 h. Vehicle and positive control were 1% DMSO and 1% Triton X-100, respectively. Then, 25 µL of Cell Titer-Glo® reagent (Promega, USA) was added to all the wells and luminescence was recorded using luminometer (SpectraMax® i3x; SoftMax® Pro Software). Percentage cytotoxicity was calculated with reference to the negative control.
Percentage of cytotoxicity was calculated with reference to the negative control cells as below:
Specific selectivity index (SSI) of ivermectin against T. evansi in comparison to mammalian cells at respective IC50 concentration was calculated using the formula:
RNA extraction from HMI-9 cultured T. evansi
Total RNA from 1 to 2 × 107 HMI-9 adapted T. evansi was isolated in triplicate using the RNeasy mini kit (Qiagen, Germany) according to the manufacturer’s instruction. The RNA of T. evansi cultured prior to addition of ivermectin was used as a control. Further, T. evansi culture medium was collected after 12, 24, and 48 h of addition of IC50 of the ivermectin to study the alteration in mRNA expression of targeted genes. DNase I (Thermo Fisher Scientific, USA) was used to remove the traces of genomic DNA. The yield and purity of RNA isolated were assessed using a NanoDrop spectrophotometer (Eppendorf, Biophotometer Plus Spectrophotometer, Germany) and diluted to 100 ng/µL concentration for the reverse transcription. The primer sequence of gene of interest was designed using Primer 3 tool (NCBI, USA), whereas the primer sequence of β-tubulin (housekeeping gene) was adopted from Brenndörfer and Boshart (2010). The list of primers of genes targeted in the present study is compiled in Table 1.
Transcript analysis
The analysis of RNA transcript of 13 gene of interest was performed using quantitative reverse transcription PCR (qRT-PCR) using Power SYBR Green RNA to Ct One Step kit (Thermo Fisher Scientific, USA) on the BioRad CFX96 Real-Time PCR Detection System (BioRad, USA). Each reaction was amounted to 10 μl volume using 1 μl (100 ng) RNA, 5 μl (2 × Power SYBR™ Green RT‑PCR Mix), 0.08 μl (125 × RT Enzyme Mix), and 100 nM each of gene-specific primer (Table 1). The fold expression of mRNA of 13 drug target genes relative to normalizer (β-tubulin) gene was calculated by ddCt method (Livak and Schmittgen 2001). The following thermocycling conditions were used: 48 °C for 30 min for cDNA synthesis, one cycle of initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 58 °C for 1 min with a single fluorescence measurement. Specificity of the PCR products was confirmed by analysis of the melt curve. The dissociation curve analysis was carried out between 60 and 95 °C with a heating rate of 0.1 °C/s and a continuous fluorescence measurement to confirm the specific amplification.
Statistical analysis
Anti-trypanosomal activity of ivermectin against T. evansi at 24, 48, and 72 h was computed by two-way ANOVA post hoc Bonferroni test. The p values < 0.05 were considered as statistically significant differences between ivermectin-treated wells and control wells. The non-linear regression analysis of ivermectin concentration and its cytotoxicity was accessed by curve fitting technique in GraphPad Prism version 8.0.2.
Result
In vitro growth inhibitory efficacy of ivermectin against T. evansi
The in vitro growth inhibitory effects of different concentrations of ivermectin against T. evansi were analyzed by two-way ANOVA post hoc Bonferroni test for comparison between the treated and control groups (Figs. 1, 2). The highly significant (p < 0.001) growth inhibition of T. evansi was observed at 24 and 48 h at higher concentration of ivermectin (20 µM and above), whereas at 72 h of in vitro treatment with ivermectin concentration of 1 µM and above, revealed highly significant (p < 0.001) difference from respective control wells. The minimal 50% inhibitory concentration (IC50) of ivermectin was calculated as 13.82 µM against T. evansi parasite by curve fit technique.
Dose-dependent effect of ivermectin on the proliferation of Trypanosoma evansi. The trypanosomes were cultured in HMI-9 medium and exposed to ivermectin for 72 h. The 24 h data was analyzed by curve fitting technique to compute minimal 50% inhibitory concentration (IC50) of ivermectin against T. evansi
Vero cell line and equine PBMCs cytotoxicity assays
Various concentrations of ivermectin (13, 26, 65, 130, and 260 µM) were tested for cytotoxicity on Vero cell line and equine PBMCs. At IC50, ivermectin showed < 20% and < 30% cytotoxicity on Vero cell line and equine PBMCs, respectively (Fig. 3). The extrapolated minimal 50% cytotoxic concentration calculated by non-linear regression analysis was 17.48 μM and 22.05 μM against Vero cell line and equine PBMCs, respectively. The SSI of ivermectin was calculated as 1.26 and 1.59 on Vero cell line and equine PBMCs, respectively, indicative low safety of the drugs for in vivo trials at this dose.
Transcript analysis
At 12 h, there is a significant down-regulation of hexokinase (p < 0.001), ESAG8 (p < 0.001), aurora kinase (p < 0.001), casein kinase 1 (p < 0.001), topoisomerase II (p < 0.001), calcium ATPase 1 (p < 0.001), and ornithine decarboxylase (p < 0.01). The down-regulation of hexokinase, aurora kinase, casein kinase 1 (p < 0.001), and calcium ATPase 1 (p < 0.001) remains continued at 24 h interval (Fig. 4). However, topoisomerase II expression gets normalized at 24 h of exposure. The arginine kinase 1 up-regulation was observed at 24 h and 48 h of treatment with ivermectin. The mRNA expression of oligopeptidase B remains refractory to the exposure of the ivermectin. All genes except ribonucleotide reductase I, casein kinase 1, and oligopeptidase B showed up-regulation in T. evansi population surviving at 48 h of exposure with drug. However, the mRNA expression of casein kinase I gets normalized at 48 h of exposure. The mRNA expression of ribonucleotide reductase I showed significant (p < 0.05) down-regulation at 24 h and 48 h of exposure with ivermectin. The trans-sialidase gene was 8.234 times more expressed at 48 h (p < 0.001), whereas it was down-regulated at 24 h (p < 0.001). On the other hand, the ribonucleotide reductase II gets significantly (p < 0.05) up-regulated at 48 h of exposure with drug.
qPCR analysis of mRNA expression kinetics of a hexokinase; b trans-sialidase; c trypanothine reductase; d ESAG8; e aurora kinase; f oligopeptidase B; g casein kinase 1; h aurora kinase; i topoisomerase II; j calcium ATPase 1; k ribonucleotide reductase I; l ribonucleotide reductase II; and m ornithine decarboxylase after treatment with IC50 of ivermectin for 48 h. The values are expressed as relative quantity with respect to the control
Discussion
Macrocyclic lactones are polyene compounds derived from Streptomycetaceae family of Actinobacteria (Hamilton-Miller, 1973; Prichard et al. 2012). Of these, ivermectin was the first macrocyclic lactone to be developed as a semi-synthetic chemically reduced 22,23-dihydro derivative of avermectin B1 produced by Streptomyces avermitilis (Campbell et al. 1983). Ivermectin has showed multifaceted anti-parasitic activity against helminth, arthropod, and protozoa parasites (Crump 2017). In the present study, ivermectin showed trypanocidal activity against T. evansi grown in the axenic culture with 50% minimal inhibitory concentration of 13.82 µM and selectivity index ranging from 1.26 to 1.59 against mammalian cells. Recently, IC50 values for ivermectin against B. bovis, B. bigemina, B. divergens, B. caballi, and T. equi were estimated as 53.3, 98.6, 30.1, 43.7, and 90.1 μM, respectively, which is several folds higher than the trypanocidal activity of the drug (Batiha et al. 2019).
Gene expression fluctuations in the presence of drug underlies the cellular decision-making process to promote the survival of cell. Target genes were selected based upon their potent role in survival of kinetoplastid parasite. Of the targeted genes, hexokinase, arginine kinases 1, and casein kinase 1 maintain the energy homeostasis, whereas ornithine decarboxylase and trypanothine reductase are vital for redox homeostasis in cell. Trans-sialidase, ESAG8, and calcium ATPase I play a major role in cellular trafficking of macromolecules. Likewise, aurora kinase, ribonucleotide reductase, and topoisomerase are necessary for DNA synthesis, replication, and cellular proliferation (Kumar et al. 2016). Data generated showed that IC50 of ivermectin significantly (p < 0.05) down-regulate the mRNA expression hexokinase, whereby, it reduces the glycolysis and energy production in T. evansi cells. Further, down-regulation of aurora kinase, ornithine decarboxylase, casein kinase 1, ribonucleotide reductase I, calcium ATPase I, and topoisomerase II hinders the proliferation of T. evansi. The mRNA expression of oligopeptidase B remains unchanged, and thereby, no reduction in virulence can be expected in T. evansi-infected host on treatment with ivermectin. Real-time qPCR analysis showed reduced expression of trans-sialidase at 24 h of exposure with ivermectin, suggesting the potential of drug to inhibit the trans-sialidase, which is indispensable for survival of the parasite. The enzyme is located in the surface of the trypanosomes and helps in accumulation of sialic acid, which cannot be synthesized by the parasite and necessary for proliferation of trypanosomes (Kumar et al. 2016). However, the T. evansi managed to eightfold enhance the expression level of trans-sialidase in comparison to control at 48 h of exposure with ivermectin in order to maintain the cellular uptake of necessary macromolecules. Similarly, ivermectin exposed T. evansi showed gradual rise in the mRNA expression of topoisomerase II at 24 h and 48 h to maintain the normal pace of DNA synthesis. Sarcoplasmic/Endoplasmic Reticulum Calcium-ATPase (SERCA) played a key role in maintenance of calcium gradient in cell and its down-regulation could be lethal to the cells (Guerrero-Hernandez et al. 2010). The overexpression of the arginine kinase in T. cruzi improves the stress-bearing ability of cells (Pereira et al. 2003). Therefore, up-regulation of arginine kinase 1 recorded in the present study may help the parasite in survival in the presence of ivermectin. The majority metabolic genes get up-regulated at 48 h interval, which must have played a key role in survival of parasites to the exposure of ivermectin. The ivermectin has demonstrated its antitumor effects on numerous cancers by targeting chloride channel, poly-ubiquitination of kinase (PAK1), multidrug resistance (MDR) gene, helicase, TOR pathway, oxidative phosphorylation pathway, ATP-dependent calcium ATPase, and many other receptors and signaling pathways (Li and Zhan 2020). Similarly, in the present study, ivermectin was found to affect glycolytic pathways, ATP-dependent calcium ATPase, cellular kinases, and other pathway involved in proliferation and maintenance of internal homeostasis of T. evansi.
In the recent COVID-19 pandemic, ivermectin has been considered as potential drug molecule against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) with IC50 value at 2 µM under in vitro conditions (Caly et al. 2020). However, the conditions under which the virus replicates and infects cells in vivo and in vitro differ, it was impossible to make a definitive statement regarding the efficacy of ivermectin in human patients. Similarly, any differences in this medication’s pharmacokinetic features, as well as any undiscovered drug interactions that may occur under such circumstances, have yet to be detected and noted. Later, it was realized that IC50 recorded against in vitro cultivated SARS-CoV-2 virus was 35 times higher than peak plasma concentration (Cmax) achieved in humans after oral administration of the approved dose of ivermectin, and therefore, the drug has low probability to produce complete cure in a clinical case (Schmith et al. 2020). Similarly, in this study, trypanocidal activity of ivermectin against T. evansi has been demonstrated IC50 at 13.82 µM under in vitro conditions; however, the results under clinical condition still need to be investigated. So far, there are few preliminary random in vivo trials (at the dose rate of 200–400 µg/kg, intraperitoneally), without determining the in vitro efficacy, of ivermectin against kinetoplastid protozoan parasites (Dias et al. 2005; Udensi and Fagbenro-Beyioku 2012; Osondu et al. 2016). Additionally, the T. evansi is responsible for nervous sign in several animal host due to its capability to invade central nervous system of host; however, ivermectin is unable to invade the blood–brain barrier (Edwards 2003) and will certainly remain ineffective in cases showing the nervous sign due to trypanosomosis. Further, the T. evansi is nowadays considered as subspecies of T. brucei (Cuypers et al. 2017; Oldrieve et al. 2021) and the ivermectin was found ineffective to treat T. brucei infection in rats (Osondu et al. 2016). Majority of these studies remain inconclusive in dearth of information regarding the effective dose required for trypanocidal activity of ivermectin. Therefore, the present study adds the information, which would be helpful in decision-making process, while attempting in vivo trials for treatment of T. evansi infection using ivermectin as a drug molecule. Further, repurposing medications such as ivermectin for management of surra infection is a promising method, but it can only be implemented if product safety has been confirmed and repurposed drug tests have been carried out at clinically relevant dosages.
Conclusions
In vitro T. evansi growth inhibitory efficacy of ivermectin was investigated. Ivermectin significantly arrested the in vitro growth of T. evansi with IC50 value at 13.82 µM. However, drug showed low specific selectivity index against Vero cells and equine PBMCs. It can be inferred that macrocyclic lactone may serve as potential drug molecule against T. evansi using nano-formulations, nano-delivery approach, and modification of structural homologs of ivermectin with varied functional groups to reduce cytotoxicity and increase bioavailability. Further, other macrocyclic lactones must also be investigated for their activity against T. evansi.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Aregawi, W.G., Agga, G.E., Abdi, R.D., Büscher, P. (2019). Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasites & Vectors, 12, 1-25. https://doi.org/10.1186/s13071-019-3311-4
Baltz, T., Baltz, D., Giroud, C., Crockett, J. (1985). Cultivation in a semi‐defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. The EMBO Journal, 4, 1273-1277.
Batiha, G.E.S., Beshbishy, A.M., Tayebwa, D.S., Adeyemi, O.S., Yokoyama, N., Igarashi, I. (2019). Evaluation of the inhibitory effect of ivermectin on the growth of Babesia and Theileria parasites in vitro and in vivo. Tropical Medicine and Health, 47, 1-12. https://doi.org/10.1186/s41182-019-0171-8
Brenndörfer, M., Boshart, M. (2010). Selection of reference genes for mRNA quantification in Trypanosoma brucei. Molecular and Biochemical Parasitology, 172, 52-55. https://doi.org/10.1016/j.molbiopara.2010.03.007
Brooks P., Grace R. (2002). Ivermectin is better than benzyl benzoate for childhood scabies in developing countries. Journal of Paediatrics Child Health, 38, 401-404. https://doi.org/10.1046/j.1440-1754.2002.00015.x.
Caly, L., Druce, J.D., Catton, M.G., Jans, D.A., Wagstaff, K.M. (2020). The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research, 178, 104787. https://doi.org/10.1016/j.antiviral.2020.104787
Campbell, W.C., Fisher, M.H., Stapley, E.O., Albers-Schönberg, G., Jacob, T.A. (1983). Ivermectin: a potent new antiparasitic agent. Science, 221, 823-828. https://doi.org/10.1126/science.6308762
Canga, A.G., Prieto, A.M.S., Liébana, M.J.D., Martínez, N.F., Vega, M.S., Vieitez, J.J.G. (2008). The pharmacokinetics and interactions of ivermectin in humans—a mini-review. The AAPS journal, 10, 42-46. https://doi.org/10.1208/s12248-007-9000-9
Crump, A. (2017). Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. The Journal of Antibiotics, 70, 495–505. https://doi.org/10.1038/ja.2017.11
Cuypers, B., Van den Broeck, F., Van Reet, N., Meehan, C.J., Cauchard, J., Wilkes, J.M., Deborggraeve, S. (2017). Genome-wide SNP analysis reveals distinct origins of Trypanosoma evansi and Trypanosoma equiperdum. Genome Biology and Evolution, 9, 1990-1997. https://doi.org/10.1093/gbe/evx102
Desquesnes, M., Holzmuller, P., Lai, D.H., Dargantes, A., Lun, Z.R., Jittaplapong, S. (2013). Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. BioMed Research International. https://doi.org/10.1155/2013/194176
Dias, J.C.P., Schofield, C.J., Machado, E.M., Fernandes, A.J. (2005). Ticks, ivermectin, and experimental Chagas disease. Memórias do Instituto Oswaldo Cruz, 100, 829-832. https://doi.org/10.1590/s0074-02762005000800002
Dou, Q., Chen, H.N., Wang, K., Yuan, K., Lei, Y., Li, K., Lan, J., Chen, Y., Huang, Z., Xie, N., Zhang, L. (2016). Therapeutics, targets, and chemical biology: ivermectin induces cytostatic autophagy by blocking the PAK1/Akt Axis in breast cancer. Cancer Research, 76, 4457-4469. https://doi.org/10.1158/0008-5472.CAN-15-2887
Edwards, G. (2003). Ivermectin: does P-glycoprotein play a role in neurotoxicity?. Filaria Journal, 2, S8. https://doi.org/10.1186/1475-2883-2-S1-S8
Giordani, F., Morrison, L.J., Rowan, T.G., De Koning, H.P., Barrett, M.P. (2016). The animal trypanosomiases and their chemotherapy: a review. Parasitology, 143, 1862-1889. https://doi.org/10.1017/S0031182016001268
Guerrero-Hernandez, A., Dagnino-Acosta, A., Verkhratsky, A. (2010). An intelligent sarco-endoplasmic reticulum Ca2+ store: release and leak channels have differential access to a concealed Ca2+ pool. Cell Calcium, 48, 143-149. https://doi.org/10.1016/j.ceca.2010.08.001
Hirumi, H., Hirumi, K. (1989). Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. The Journal of Parasitology, 985–989.
Kumar, R., Singh, J., Singh, R., Kumar, S., Yadav, S. C. (2015). Comparative efficacy of different in vitro cultivation media for Trypanosoma evansi isolated from different mammalian hosts inhabiting different geographical areas of India. Journal of Parasitic Diseases, 39, 174-178. https://doi.org/10.1007/s12639-013-0314-5
Kumar, R., Sharma, P., Kumar Gaur, D., & Jain, S. (2016). Recent development in identification of potential novel therapeutic targets against trypanosomatids. Current Topics in Medicinal Chemistry, 16, 2303-2315. https://doi.org/10.2174/1568026616666160413125453
Kumar, R., Rani, R., Kumar, S., Sethi, K., Jain, S., Batra, K., Kumar S., Tripathi, B.N. (2020). Drug-induced reactive oxygen species–mediated inhibitory effect on growth of Trypanosoma evansi in axenic culture system. Parasitology Research, 119, 3481-3489. https://doi.org/10.1007/s00436-020-06861-7
Li, N., Zhan, X. (2020). Anti-parasite drug ivermectin can suppress ovarian cancer by regulating lncRNA-EIF4A3-mRNA axes. EPMA Journal, 11, 289-309. https://doi.org/10.1007/s13167-020-00209-y
Livak, K.J., Schmittgen, T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods, 25, 402-408. https://doi.org/10.1006/meth.2001.1262
Mendes A.M., Albuquerque I.S., Machado M., Pissarra J., Meireles P., Prudêncio M. (2017). Inhibition of Plasmodium liver infection by ivermectin. Antimicrobial Agents and Chemotherapy, 61, e02005–e02016. https://doi.org/10.1128/AAC.02447-16.
Oldrieve, G., Verney, M., Jaron, K. S., Hébert, L., & Matthews, K. R. (2021). Monomorphic Trypanozoon: towards reconciling phylogeny and pathologies. Microbial Genomics, 7https://doi.org/10.1099/mgen.0.000632
Ōmura S, Crump A. (2017). Ivermectin and malaria control. Malaria Journal, 16, 172. https://doi.org/10.1186/s12936-017-1825-9.
Omura S, Crump A. (2014). Ivermectin: panacea for resource-poor communities? Trends in Parasitology, 30, 445-455. https://doi.org/10.1016/j.pt.2014.07.005.
Osondu, F., Ugochukwu, C.I.I., Ugochukwu, E.I. (2016). A comparative study of the chemotherapeutic effects of diminazene aceturate and Ivermectin on Trypanosoma brucei brucei infected rats. Asian Pacific Journal of Tropical Disease, 6, 341-346.
Panchal, M., Rawat, K., Kumar, G., Kibria, K.M., Singh, S., Kalamuddin, M., Mohmmed, A., Malhotra, P., Tuteja, R. (2014). Plasmodium falciparum signal recognition particle components and anti-parasitic effect of ivermectin in blocking nucleo-cytoplasmic shuttling of SRP. Cell death and Disease. 5, e994. https://doi.org/10.1038/cddis.2013.521
Pereira, C.A., Alonso, G.D., Ivaldi, S., Silber, A.M., Alves, M.J.M., Torres, H.N., Flawiá, M.M. (2003). Arginine kinase overexpression improves Trypanosoma cruzi survival capability. FEBS Letters, 554, 201-205. https://doi.org/10.1016/s0014-5793(03)01171-2
Pinilla, Y.T., Lopes, S.C.P., Sampaio, V.S., Andrade, F.S., Melo, G.C., Orfanó, A.S., et al. (2018) Promising approach to reducing malaria transmission by ivermectin: sporontocidal effect against Plasmodium vivax in the south American vectors Anopheles aquasalis and Anopheles darlingi. PLoS Neglected Tropical Disease, 12, e0006221. https://doi.org/10.1371/journal.pntd.0006221.
Prichard, R., Ménez, C., Lespine, A. (2012). Moxidectin and the avermectins: consanguinity but not identity. International Journal for Parasitology: Drugs and Drug Resistance, 2, 134-153. https://doi.org/10.1016/j.ijpddr.2012.04.001
Pushpakom, S., Iorio F., Eyers, P.A., Escott, K.J., Hopper, S., Wells, A., Doig, A., Guilliams, T., Latimer, J., McNamee, C., Norris, A. (2019). Drug repurposing: progress, challenges and recommendations. Nature Reviews Drug discovery, 18, 41-58. https://doi.org/10.1038/nrd.2018.168
Radwanska, M., Vereecke, N., Deleeuw, V., Pinto, J., Magez, S. (2018). Salivarian trypanosomosis: a review of parasites involved, their global distribution and their interaction with the innate and adaptive mammalian host immune system. Frontiers in Immunology, 9, 2253. https://doi.org/10.3389/fimmu.2018.02253
Reis, T.A., Oliveira-da-Silva, J.A., Tavares, G.S., Mendonça, D.V., Freitas, C.S., Costa, R.R., Lage, D.P., Martins, V.T., Machado, A.S., Ramos, F.F., Silva, A.M. (2021). Ivermectin presents effective and selective anti-leishmanial activity in vitro and in vivo against Leishmania infantum and is therapeutic against visceral leishmaniasis. Experimental Parasitology. 221, 108059. https://doi.org/10.1016/j.exppara.2020.108059
Rudrapal, M., Khairnar, J.S., Jadhav, G.A. (2020). Drug repurposing (DR): an emerging approach in drug discovery. Drug Repurposing - Hypothesis, Molecular Aspects and Therapeutic Applications https://doi.org/10.5772/intechopen.93193
Schmith, V.D., Zhou, J., Lohmer, L.R. (2020). The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID‐19. Clinical Pharmacology and Therapeutics, 108, 762-765. https://doi.org/10.1002/cpt.1889
Sebaugh, J.L. (2011). Guidelines for accurate EC50/IC50 estimation. Pharmaceutical statistics, 10(2), 128-134. https://doi.org/10.1002/pst.426
Sharma, D., Gupta, S., Sethi, K., Kumar, S., Kumar, R. (2022). Seroprevalence and immunological characterization of Trypanosoma evansi infection in livestock of four agro-climatic zones of Himachal Pradesh, India. Tropical Animal Health and Production, 54, 1-10. https://doi.org/10.1007/s11250-022-03069-y.
Udensi, U.K., Fagbenro-Beyioku, A.F. (2012). Effect of ivermectin on Trypanosoma brucei brucei in experimentally infected mice. Journal of Vector Borne Diseases, 49, 143. https://doi.org/10.1002/cpt.1889
Yadav, S.C., Kumar, P., Khurana, S., Kumar, R. (2019). Seroprevalence of Trypanosoma evansi infection in equines of north and north western states of india. Journal of Equine Veterinary Science, 79, 63-67. https://doi.org/10.1016/j.jevs.2019.05.019
Acknowledgements
The authors gratefully thank the Director, ICAR-National Research Centre on Equines, Hisar, India, for providing all the necessary facilities for conducting this study. The authors are thankful to Mr. Raj Kumar Dayal for technical assistance provided during the experiments.
Funding
The financial support from ICAR, New Delhi is duly acknowledged.
Author information
Authors and Affiliations
Contributions
RK, SV, BCB, and SK designed the research proposal. SG, SG, and KS conceived the research and prepared the final draft of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Prior approval was taken for animal experimentation in the present study from Institutional Animal Ethics Committee of ICAR-NRCE, Hisar.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, S., Vohra, S., Sethi, K. et al. In vitro anti-trypanosomal effect of ivermectin on Trypanosoma evansi by targeting multiple metabolic pathways. Trop Anim Health Prod 54, 240 (2022). https://doi.org/10.1007/s11250-022-03228-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03228-1