Abstract
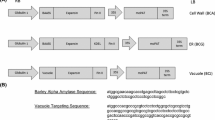
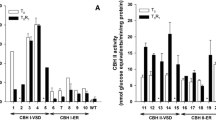
Cellobiohydrolase II (CBH II) is an exo-glucanase that is part of a fungal mixture of enzymes from a wood-rot fungus, Trichoderma reesei. It is therefore difficult to purify and to establish a specific activity assay. The gene for this enzyme, driven by the rice Os glutelin promoter, was transformed into High II tissue culture competent corn, and the enzyme accumulated in the endosperm of the seed. The transgenic line recovered from tissue culture was bred into male and female elite Stine inbred corn lines, stiff stalk 16083-025 (female) and Lancaster MSO411 (male), for future production in their hybrid. The enzyme increases its accumulation throughout its 6 generations of back crosses, 27–266-fold between T1 and T2, and 2–10-fold between T2 and T3 generations with lesser increases in T4–T6. The germplasm of the inbred lines replaces the tissue culture corn variety germplasm with each generation, with the ultimate goal of producing a high-yielding hybrid with the transgene. The CBH II enzyme was purified from T5 inbred male grain 10-fold to homogeneity with 47.5% recovery. The specific activity was determined to be 1.544 units per µg protein. The corn-derived CBH II works in biopolishing of cotton by removing surface fibers to improve dyeability and increasing glucose from corn flour for increasing ethanol yield from starch-based first-generation processes.





Similar content being viewed by others
References
Ander P, Hildén L, Daniel G (2008) Cleavage of softwood kraft pulp fibres by HCl and cellulases. BioResources 3:477–490
Andreaus J, Olekszyszen DN, Silveria M (2014) Processing of cellulosic textile materials with cellulases. Cellulose and other naturally occurring polymers. Research Signpost, Kerala, pp 11–19
Armstrong C, Green C, Phillips R (1991) Development and availability of germplasm with high type II culture formation response. Maize Genet Coop Newslett 65:92–93
Csiszar E, Urbánszki K, Szakacs G (2001) Biotreatment of desized cotton fabric by commercial cellulase and xylanase enzymes. J Mol Catal B Enzym 11:1065–1072
Devaiah SP, Requesens DV, Chang Y-K, Hood KR, Flory A, Howard JA, Hood EE (2013) Heterologous expression of cellobiohydrolase II (Cel6A) in maize endosperm. Transgenic Res 22:477–488
Ferreira RDG, Azzoni AR, Freitas S (2018) Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: the case of recombinant β-glucosidase. Biotechnol Biofuels 11(81):1–13
Hettenhaus J (2011) Agricultural crop residues for biomass conversion. In: Hood E, Nelson P, Powell R (eds) Plant biomass conversion. Wiley-Blackwell, Ames, pp 21–50
Hood E, Love R, Lane J, Bray J, Clough R, Pappu K, Drees C, Hood K, Yoon S, Ahmad A (2007) Subcellular targeting is a key condition for high-level accumulation of cellulase protein in transgenic maize seed. Plant Biotechnol J 5:709–719
Hood EE, Devaiah SP, Fake G, Egelkrout E, Teoh K, Requesens DV, Hayden C, Hood KR, Pappu KM, Carroll J, Howard JA (2012) Manipulating corn germplasm to increase recombinant protein accumulation. Plant Biotechnol J 10:20–30
Hood NC, Hood KR, Woodard SL, Devaiah SP, Jeoh T, Wilken L, Nikolov Z, Egelkrout E, Howard JA, Hood EE (2014) Purification and characterization of recombinant Cel7A from maize seed. Appl Biochem Biotechnol 174:2864–2874
Howard JA, Nikolov Z, Hood EE (2011) Enzyme production systems for biomass conversion. In: Hood EE, Nelson P, Powell R (eds) Plant biomass conversion. Wiley, NJ, pp 227–253
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109:1083–1087
Liu G, Zhang J, Bao J (2016) Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess Biosyst Eng 39:133–140
Ng IS, Wu X, Lu Y, Yao C (2014) Trichoderma reesei cellulase complex in hydrolysis of agricultural waste of grapefruit peel and orange peel. BioResources 9:6420–6431
Saloheimo M, Pakula TM (2012) The cargo and the transport system: secreted proteins and protein secretion in Trichoderma reesei (Hypocrea jecorina). Microbiology 158:46–57
Saravanan D, Vasanthi N, Ramachandran T (2009) A review on influential behaviour of biopolishing on dyeability and certain physico-mechanical properties of cotton fabrics. Carbohyd Polym 76:1–7
Šimić K, Soljačić I, Pušić T (2015) Application of cellulases in the process of finishing. Tekstilec 58:47–56
Takaiwa F, Kikuchi S, Oono K (1987) A rice glutelin gene family—a major type of glutelin mRNAs can be divided into two classes. Mol Gen Genet MGG 208:15–22
Tavcer PF (2013) Effects of cellulase enzyme treatment on the properties of cotton terry fabrics. Fibres Text East Eur 6(102):100–106
Teeri TT, Lehtovaara P, Kauppinen S, Salovuori I, Knowles J (1987) Homologous domains in Trichoderma reesei cellulolytic enzymes: gene sequence and expression of cellobiohydrolase II. Gene 51:43–52
Uddin MG (2015) Effects of biopolishing on the quality of cotton fabrics using acid and neutral cellulases. Text Cloth Sustain 1:1–10
Wu C-Y, Adach T, Hatano T, Washida H, Suzuki A, Takaiwa F (1998) Promoters of rice seed storage protein genes direct endosperm-specific gene expression in transgenic rice. Plant Cell Physiol 39:885–889
Author information
Authors and Affiliations
Contributions
ED and MAS—performed laboratory work used in the manuscript. NH—did field work and pollinations. EEH—analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors are employees of the company which sponsored the research for this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duque, E.D.Y., Aguirre, M., Hood, N.C. et al. Specific activity and utility of recombinant cellobiohydrolase II (Cel6A) produced in maize endosperm. Transgenic Res 33, 47–57 (2024). https://doi.org/10.1007/s11248-024-00376-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-024-00376-y