Abstract
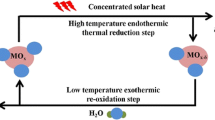
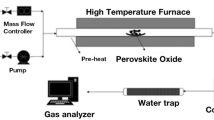
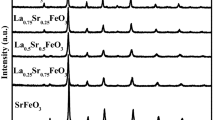
Mixed oxides with perovskite structure have been proposed as promising alternative for the solar fuel production via thermochemical redox cycles. For this work, the system La0.6Sr0.4Mn1−xAlxO3 (x = 0 to 0.8) was selected according to its high thermal stability and rapid oxidation kinetics, and the influence of the Al/Mn ratio on the redox properties was investigated. The characterization of the five oxides samples with different Al content confirmed the high redox capacity and the favorable behavior of these materials in consecutive cycles, as analyzed thermogravimetrically. The results show that following reduction at 1300 °C in inert atmosphere up to 0.32 mmol g−1 of O2 are released, while a 10-cycle reaction test confirms the feasibility of long term operation with these perovskites. It was observed that the reduction extent was enhanced with increasing the Al-content, but the oxidation degree is maximum for compositions near x = 0.5, corresponding to an O2 release of 0.318 mmol g−1 (δ = 0.132). After selecting the compositions with more promising redox properties, additional reactions were performed in a lab-scale fixed bed reactor with injection of CO2 in the oxidation step at 900 °C in order to generate CO. In these tests, the most interesting results were obtained for the perovskite La0.6Sr0.4Mn0.6Al0.4O3, with reduction extent of 0.266 mmol g−1, but the production of CO is in comparison significantly lower (0.114 mmol g−1). Further studies are required to determine the best operation conditions for thermochemical cycles using those materials.













Similar content being viewed by others
References
Steinfeld A, Palumbo R (2003) Solar thermochemical process technology. In: Encyclopedia of physical science and technology. Elsevier, Amsterdam, pp 237–256
Serpone N, Lawless D, Terzian R (1992) Solar fuels: status and perspectives. Sol Energy 49:221–234. doi:10.1016/0038-092X(92)90001-Q
Abanades S, Charvin P, Lemont F, Flamant G (2008) Novel two-step SnO2/SnO water-splitting cycle for solar thermochemical production of hydrogen. Int J Hydrog Energy 33:6021–6030. doi:10.1016/j.ijhydene.2008.05.042
Agrafiotis C, Roeb M, Sattler C (2015) A review on solar thermal syngas production via redox pair-based water/carbon dioxide splitting thermochemical cycles. Renew Sustain Energy Rev 42:254–285. doi:10.1016/j.rser.2014.09.039
Allendorf MD, Diver RB, Siegel NP, Miller JE (2008) Two-step water splitting using mixed-metal ferrites: thermodynamic analysis and characterization of synthesized materials. Energy Fuels 22:4115–4124. doi:10.1021/ef8005004
Kodama T, Kondoh Y, Yamamoto R et al (2005) Thermochemical hydrogen production by a redox system of ZrO2-supported Co(II)-ferrite. Sol Energy 78:623–631. doi:10.1016/j.solener.2004.04.008
Kuhn M, Bishop SR, Rupp JLM, Tuller HL (2013) Structural characterization and oxygen nonstoichiometry of ceria-zirconia (Ce1−xZrxO2−δ) solid solutions. Acta Mater 61:4277–4288. doi:10.1016/j.actamat.2013.04.001
Marugán J, Botas JA, Martín M et al (2012) Study of the first step of the Mn2O3/MnO thermochemical cycle for solar hydrogen production. Int J Hydrog Energy 37:7017–7025. doi:10.1016/j.ijhydene.2011.10.124
Nakamura T (1977) Hydrogen production from water utilizing solar heat at high temperatures. Sol Energy 19:467–475. doi:10.1016/0038-092X(77)90102-5
Scheffe JR, Li J, Weimer AW (2010) A spinel ferrite/hercynite water-splitting redox cycle. Int J Hydrog Energy 35:3333–3340. doi:10.1016/j.ijhydene.2010.01.140
Scheffe JR, McDaniel AH, Allendorf MD, Weimer AW (2013) Kinetics and mechanism of solar-thermochemical H2 production by oxidation of a cobalt ferrite–zirconia composite. Energy Environ Sci 6:963. doi:10.1039/c3ee23568h
Scheffe JR, Steinfeld A (2014) Oxygen exchange materials for solar thermochemical splitting of H2O and CO2: a review. Mater Today 17:341–348. doi:10.1016/j.mattod.2014.04.025
Steinfeld A (2002) Solar hydrogen production via a two-step water-splitting thermochemical cycle based on Zn/ZnO redox reactions. Int J Hydrog Energy 27:611–619. doi:10.1016/S0360-3199(01)00177-X
Steinfeld A, Meier A (2004) Solar fuels and materials. Encycl. Energy 5:15. doi:10.1016/B0-12-176480-X/00322-3
Xiao L, Wu S-Y, Li Y-R (2012) Advances in solar hydrogen production via two-step water-splitting thermochemical cycles based on metal redox reactions. Renew Energy 41:1–12. doi:10.1016/j.renene.2011.11.023
Michalsky R, Botu V, Hargus CM et al (2015) Design principles for metal oxide redox materials for solar-driven isothermal fuel production. Adv Energy Mater 5:1–10. doi:10.1002/aenm.201401082
Scheffe JR, Steinfeld A (2012) Thermodynamic analysis of cerium-based oxides for solar thermochemical fuel production. Energy Fuels 26:1928–1936. doi:10.1021/ef201875v
Scheffe JR, Jacot R, Patzke GR, Steinfeld A (2013) Synthesis, characterization, and thermochemical redox performance of Hf4+, Zr4+, and Sc3+ doped ceria for splitting CO2. J Phys Chem C 117:24104–24114. doi:10.1021/jp4050572
Hao Y, Yang C-K, Haile SM (2014) Ceria–Zirconia solid solutions (Ce1–xZrxO2–δ, x ≤ 0.2) for solar thermochemical water splitting: a thermodynamic study. Chem Mater 26:6073–6082. doi:10.1021/cm503131p
Kang M, Zhang J, Wang C et al (2013) CO2 splitting via two step thermochemical reactions over doped ceria/zirconia solid solutions. RSC Adv 3:18878–18885. doi:10.1039/C3RA43742F
Ramos-Fernandez E V., Shiju NR, Rothenberg G (2014) Understanding the solar-driven reduction of CO2 on doped ceria. RSC Adv 4:16456. doi:10.1039/c4ra01242a
Marxer D, Furler P, Scheffe J et al (2015) Demonstration of the entire production chain to renewable kerosene via solar thermochemical splitting of H2O and CO2. Energy Fuels 29:3241–3250. doi:10.1021/acs.energyfuels.5b00351
Takacs M, Hoes M, Caduff M et al (2016) Oxygen nonstoichiometry, defect equilibria, and thermodynamic characterization of LaMnO3 perovskites with Ca/Sr A-site and Al B-site doping. Acta Mater 103:700–710. doi:10.1016/j.actamat.2015.10.026
Yang C-K, Yamazaki Y, Aydin A, Haile SM (2014) Thermodynamic and kinetic assessments of strontium-doped lanthanum manganite perovskites for two-step thermochemical water splitting. J Mater Chem A 2:13612–13623. doi:10.1039/C4TA02694B
Bork AH, Kubicek M, Struzik M, Rupp JLM (2015) Perovskite La0.6Sr0.4Cr1–xCoxO3–δ solid solutions for solar-thermochemical fuel production: strategies to lower the operation temperature. J Mater Chem A 3:15546–15557. doi:10.1039/C5TA02519B
Scheffe JR, Weibel D, Steinfeld A (2013) Lanthanum–Strontium–Manganese perovskites as redox materials for solar thermochemical splitting of H2O and CO2. Energy Fuels 27:4250–4257. doi:10.1021/ef301923h
Demont A, Abanades S (2014) High redox activity of Sr-substituted lanthanum manganite perovskites for two-step thermochemical dissociation of CO2. RSC Adv 4:54885–54891. doi:10.1039/C4RA10578H
Dey S, Naidu BS, Govindaraj A, Rao CNR (2015) Noteworthy performance of La1–xCaxMnO3 perovskites in generating H2 and CO by the thermochemical splitting of H2O and CO2. Phys Chem Chem Phys 17:122–125. doi:10.1039/C4CP04578E
Bork AH, Povoden-Karadeniz E, Rupp JLM (2016) Modeling thermochemical solar-to-fuel conversion: CALPHAD for thermodynamic assessment studies of perovskites, exemplified for (La,Sr)MnO3. Adv Energy Mater. doi:10.1002/aenm.201601086
Deml AM, Stevanović V, Holder AM et al (2014) Tunable oxygen vacancy formation energetics in the complex perovskite oxide SrxLa1−xMnyAl1−yO3. Chem Mater 26:6595–6602. doi:10.1021/cm5033755
Demont A, Abanades S (2015) Solar thermochemical conversion of CO2 into fuel via two-step redox cycling of non-stoichiometric Mn-containing perovskite oxides. J Mater Chem A 3:3536–3546. doi:10.1039/C4TA06655C
Dey S, Naidu BS, Rao CNR (2016) Beneficial effects of substituting trivalent ions in the B-site of La0.5Sr0.5Mn1−xAxO3 (A = Al, Ga, Sc) on the thermochemical generation of CO and H2 from CO2 and H2O. Daltan Trans 45:2430–2435. doi:10.1039/C5DT04822B
Jiang Q, Tong J, Zhou G et al (2014) Thermochemical CO2 splitting reaction with supported LaxA1–xFeyB1–yO3 (A = Sr, Ce, B = Co, Mn; 0 ≤ x, y ≤ 1) perovskite oxides. Sol Energy 103:425–437. doi:10.1016/j.solener.2014.02.033
McDaniel AH, Miller EC, Arifin D et al (2013) Sr- and Mn-doped LaAlO3–δ for solar thermochemical H2 and CO production. Energy. Environ Sci 6:2424. doi:10.1039/c3ee41372a
Nalbandian L, Evdou A, Zaspalis V (2009) La1–xSrxMO3 (M = Mn, Fe) perovskites as materials for thermochemical hydrogen production in conventional and membrane reactors. Int J Hydrog Energy 34:7162–7172. doi:10.1016/j.ijhydene.2009.06.076
Gálvez ME, Jacot R, Scheffe J et al (2015) Physico-chemical changes in Ca, Sr and Al-doped La–Mn–O perovskites upon thermochemical splitting of CO2 via redox cycling. Phys Chem Chem Phys 17:6629–6634. doi:10.1039/c4cp05898d
McDaniel AH, Ambrosini A, Coker EN et al (2013) Nonstoichiometric perovskite oxides for solar thermochemical H2 and CO production. Energy Procedi 49:2009–2018. doi:10.1016/j.egypro.2014.03.213
Pechini MP (1967) US Patent No. 3,330,697
Jana P, de la Peña O’Shea VA, Coronado JM, Serrano DP (2010) Cobalt based catalysts prepared by Pechini method for CO2-free hydrogen production by methane decomposition. Int J Hydrog Energy 35:10285–10294. doi:10.1016/j.ijhydene.2010.07.125
Cooper T, Scheffe JR, Galvez ME et al (2015) Lanthanum Manganite perovskites with Ca/Sr A-site and Al B-site doping as effective oxygen exchange materials for solar thermochemical fuel production. Energy Technol 3:1130–1142. doi:10.1002/ente.201500226
Xu B, Bhawe Y, Davis ME (2013) Spinel metal oxide-alkali carbonate-based, low-temperature thermochemical cycles for water splitting and CO2 reduction. Chem Mater 25:1564–1571. doi:10.1021/cm3038747
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A 32:751–767. doi:10.1107/S0567739476001551
Goldschmidt VM (1926) Die Gesetze der Krystallochemie. Naturwissenschaften 14:477–485. doi:10.1007/BF01507527
Dalach P, Ellis DE, Van De Walle A (2012) First-principles thermodynamic modeling of lanthanum chromate perovskites. Phys Rev B 85:1–13. doi:10.1103/PhysRevB.85.014108
Acknowledgements
The authors thank to “Ramón Areces” Foundation for financial support through the project SOLARKITE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sastre, D., Carrillo, A.J., Serrano, D.P. et al. Exploring the Redox Behavior of La0.6Sr0.4Mn1−xAlxO3 Perovskites for CO2-Splitting in Thermochemical Cycles. Top Catal 60, 1108–1118 (2017). https://doi.org/10.1007/s11244-017-0790-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-017-0790-4