Abstract
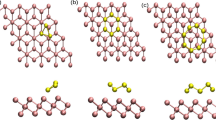
Interactions of O2 with the PdO(101) surface were studied using spin-dependent density-functional theory (DFT) with both the PBE and the non-local hybrid HSE exchange–correlation functional. The adsorption energies are strongly overestimated (by 40–60 kJ/mol) with PBE, whereas HSE predicts adsorption energies that are within ~5 kJ/mol of values derived from temperature programmed desorption (TPD) experiments. A detailed partial density of states analysis indicates that the band gap between the PdO d-band center and the LUMO of O2 plays an important role in determining the adsorption strength. This gap is larger for the HSE functional and leads to a decrease in the back donation of the metal d-states to the O2 LUMO orbital resulting in weaker adsorption. Based on the DFT–HSE calculations, three adsorption minima are found to be stable. The most favored configuration, with an adsorption energy of −67 kJ/mol, consists of an O2 molecule lying flat and interacting with two coordinatively unsaturated Pd (Pdcus) surface atoms. The other two configurations have weaker adsorption energies of about −25 kJ/mol and bind to a single Pdcus atom with the O2 molecule oriented away from the surface. The HSE results can be correlated with the observed TPD spectra, which shows only one type of O2 configuration at low coverages with a subsequently lower temperature (more weakly bound) peak evolving at higher coverages associated with the singly coordinated O2 adsorption configurations that start to populate when two adjacent Pdcus sites start to become unavailable.






Similar content being viewed by others
References
McCarty JG (1995) Catal Today 26:283–293
Datye AK, Bravo J, Nelson TR, Atanasova P, Lyubovsky M, Pfefferle L (2000) Appl Catal A 198:179–196
Carstens JN, Su SC, Bell AT (1998) J Catal 176:136–142
Hoffmann MJ, Reuter K (2014) Top Catal 57:159–170
Duan Z, Henkelman G (2014) ACS Catal 4:3435–3443
Weng X, Yuan X, Li H, Li X, Chen M, Wan H (2015) Sci China Chem 58:174–179
Martin NM, Van Den Bossche M, Grönbeck H, Hakanoglu C, Zhang F, Li T, Gustafson J, Weaver JF, Lundgren E (2014) J Phys Chem C 118:1118–1128
Hellman A, Resta A, Martin NM, Gustafson J, Trinchero A, Carlsson PA, Balmes O, Felici R, Van Rijn R, Frenken JWM, Andersen JN, Lundgren E, Grönbeck H (2012) J Phys Chem Lett 3:678–682
Martin NM, Van Den Bossche M, Hellman A, Grönbeck H, Hakanoglu C, Gustafson J, Blomberg S, Johansson N, Liu Z, Axnanda S, Weaver JF, Lundgren E (2014) ACS Catal 4:3330–3334
Blomberg S, Hoffmann MJ, Gustafson J, Martin NM, Fernandes VR, Borg A, Liu Z, Chang R, Matera S, Reuter K, Lundgren E (2013) Phys Rev Lett 110:117601
Kolasinski KW, Cemic F, Demeijere A, Hasselbrink E (1995) Surf Sci 334:19–28
Gabasch H, Knop-Gericke A, Schlögl R, Borasio M, Weilach C, Rupprechter G, Penner S, Jenewein B, Hayek K, Klötzer B (2007) Phys Chem Chem Phys 9:533–540
Toyoshima R, Yoshida M, Monya Y, Kousa Y, Suzuki K, Abe H, Mun BS, Mase K, Amemiya K, Kondoh H (2012) J Phys Chem C 116:18691–18697
Imbihl R, Demuth JE (1986) Surf Sci 173:395–410
Guo X, Hoffman A, Yates JT (1989) J Chem Phys 90:5787
Sjovall P, Uvdal P (1998) J Vac Sci Technol A 16:943–947
Eichler A, Mittendorfer F, Hafner J (2000) Phys Rev B 62:4744–4755
Honkala K, Laasonen K (2001) J Chem Phys 115:2297–2302
Campbell CT, Sellers JRV (2013) Chem Rev 113:4106–4135
Weaver JF (2013) Chem Rev 113:4164–4215
Lundgren E, Mikkelsen A, Andersen JN, Kresse G, Schmid M, Varga P (2006) J Phys 18:R481–R499
Kan HH, Weaver JF (2008) Surf Sci 602:L53–L57
Weaver JF, Hakanoglu C, Antony A, Asthagiri A (2014) Chem Soc Rev 43:7536–7547
Hinojosa JA, Kan HH, Weaver JF (2008) J Phys Chem C 112:8324–8331
Zygmunt SA, Curtiss LA (2005) Quantum-chemical studies of molecular reactivity in nanoporous materials. In: Curtiss LA, Gordon MS (eds) Computational materials chemistry. Kluwer, Dordrecht, pp 191–245
Hammer B, Hansen L, Nørskov J (1999) Phys Rev B 59:7413–7421
Liu H-R, Xiang H, Gong XG (2011) J Chem Phys 135:214702
Patton DC, Porezag DV, Pederson MR (1997) Phys Rev B 55:7454–7459
Lide DR (2013) CRC Handbook of chemistry and physics, 94th Edition, 2013–2014. CRC Press, Boca raton
Kiejna A, Kresse G, Rogal J, De Sarkar A, Reuter K, Scheffler M (2006) Phys Rev B 73:35404
Stroppa A, Termentzidis K, Paier J, Kresse G, Hafner J (2007) Phys Rev B 76:195440
Gajdos M, Eichler A, Hafner J (2004) J Phys 1141:16
Zhang F, Pan L, Li T, Diulus JT, Asthagiri A, Weaver JF (2014) J Phys Chem C 118:28647–28661
Bruska MK, Czekaj I, Delley B, Mantzaras J, Wokaun A (2011) Phys Chem Chem Phys 13:15947–15954
Marsman M, Paier J, Stroppa A, Kresse G (2008) J Phys 20:064201
Paier J, Marsman M, Hummer K, Kresse G, Gerber IC, Angyán JG (2006) J Chem Phys 124:154709
Van Den Bossche M, Martin NM, Gustafson J, Hakanoglu C, Weaver JF, Lundgren E, Grönbeck H (2014) J Chem Phys 141(3):034706
Hirvi JT, Kinnunen T-JJ, Suvanto M, Pakkanen TA, Nørskov JK (2010) J Chem Phys 133:084704
Nørskov JK, Rossmeisl J, Logadottir A, Lindqvist L, Kitchin JR, Bligaard T, Jónsson H (2004) J Phys Chem B 108:17886–17892
Van den Bossche M, Grönbeck H (2015) J Am Chem Soc 137:12035–12044
Kresse G, Hafner J (1993) Phys Rev B 47:558–561
Kresse G, Hafner J (1993) J Non Cryst Solids 156–158:956–960
Blöchl PE (1994) Phys Rev B 50:17953–17979
Kresse G, Joubert D (1999) Phys Rev B 59:1758–1775
Perdew J, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Heyd J, Scuseria GE, Ernzerhof M (2003) J Chem Phys 118:8207
Monkhorst H, Pack J (1976) Phys Rev B 13:5188–5192
Weaver JF, Hakanoglu C, Hawkins JM, Asthagiri A (2010) J Chem Phys 132:024709
Hakanoglu C, Hawkins JM, Asthagiri A, Weaver JF (2010) J Phys Chem C 114:11485–11497
Weaver JF, Hakanoglu C, Antony A, Asthagiri A (2011) J Am Chem Soc 133:16196–16200
Antony A, Hakanoglu C, Asthagiri A, Weaver JF (2012) J Chem Phys 136:054702
Kan HH, Weaver JF (2009) Surf Sci 603:2671–2682
Sheppard D, Terrell R, Henkelman G (2008) J Chem Phys 128:134106
Tang W, Sanville E, Henkelman G (2009) J Phys 21:084204
Henkelman G, Arnaldsson A, Jónsson H (2006) Comput Mater Sci 36:354–360
Jiménez-Hoyos CA, Janesko BG, Scuseria GE (2008) Phys Chem Chem Phys 10:6621–6629
Irikura KK (2007) J Phys Chem Ref Data 36:389–397
Schweitzer C, Schmidt R (2003) Chem Rev 103:1685–1758
Wang L, Maxisch T, Ceder G (2006) Phys Rev B 73:195107
Scanlon DO, Morgan BJ, Watson GW, Walsh A (2009) Phys Rev Lett 103:096405
Rogers DB, Shannon RD, Gillson JL (1971) J Solid State Chem 3:314–316
Nilsson PO (1979) J Phys C 12:1423
Okamoto H, Asô T (1967) Jpn J Appl Phys 6:779
Rey E, Kamal MR, Miles RB, Royce BSH (1978) J Mater Sci 13:812–816
Pawlas-Foryst E, Zabdyr L (2008) Arch Metall Mater 53:1173–1175
Wang H, Schneider WF, Schmidt D (2009) J Phys Chem C 113:15266–15273
Finlay RJ, Her T, Wu C, Mazur E (1997) Surface femtochemistry of oxygen and coadsorbates on Pt(111). In: Sundstrom V (ed) Femtochemistry femtobiology ultrafast react. Imperial College, London, pp 629–659
Kresse G, Gil A, Sautet P (2003) Phys Rev B 68:3–6
Hammer B, Nørskov JK (1995) Surf Sci 343:211–220
Acknowledgments
We acknowledge the Ohio Supercomputing Center for providing computational resources. We gratefully acknowledge financial support for this work provided by the Department of Energy, Office of Basic Energy Sciences, Catalysis Science Division through Grant DE-FG02-03ER15478.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, L., Weaver, J.F. & Asthagiri, A. First Principles Study of Molecular O2 Adsorption on the PdO(101) Surface. Top Catal 60, 401–412 (2017). https://doi.org/10.1007/s11244-016-0705-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-016-0705-9